Predicting precipitate formation is crucial in various fields, including chemistry, biology, and environmental science. Precipitates are insoluble solid particles that form when a solution is supersaturated with a particular compound or when two solutions containing different ions are mixed. In this article, we will explore seven ways to predict precipitate formation, including the use of solubility rules, ion product, and various mathematical models.
Understanding Precipitate Formation

Precipitate formation occurs when a solution is supersaturated with a particular compound or when two solutions containing different ions are mixed. This process is driven by the concentration of ions in the solution and the solubility of the compound. There are several factors that influence precipitate formation, including the concentration of ions, temperature, pH, and the presence of other ions.
Factors Affecting Precipitate Formation
- Concentration of ions: The concentration of ions in the solution plays a crucial role in precipitate formation. If the concentration of ions is high, the solution is more likely to be supersaturated, leading to precipitate formation.
- Temperature: Temperature affects the solubility of compounds. In general, the solubility of compounds increases with increasing temperature.
- pH: The pH of the solution affects the solubility of compounds. Some compounds are more soluble at certain pH levels.
- Presence of other ions: The presence of other ions in the solution can affect the solubility of the compound and lead to precipitate formation.
Method 1: Solubility Rules

Solubility rules are a set of guidelines used to predict whether a compound will dissolve in water or form a precipitate. These rules are based on the properties of the ions involved and the type of compound being formed. There are several solubility rules, including:
- Most sodium, potassium, and ammonium salts are soluble.
- Most nitrates and acetates are soluble.
- Most chlorides, bromides, and iodides are soluble, except for silver and lead.
- Most sulfates are soluble, except for barium, strontium, and lead.
By applying these solubility rules, you can predict whether a compound will form a precipitate when mixed with a particular solution.
Example
- Predict whether a precipitate will form when a solution of sodium chloride (NaCl) is mixed with a solution of silver nitrate (AgNO3).
- Using the solubility rules, we can see that sodium chloride is soluble, while silver chloride is insoluble.
- Therefore, a precipitate of silver chloride will form when the two solutions are mixed.
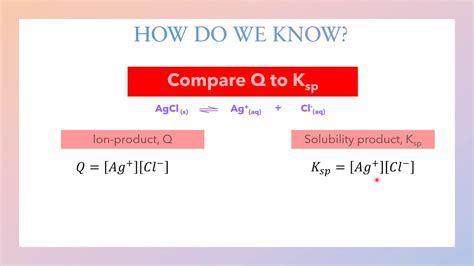
Method 2: Ion Product (Q)

The ion product (Q) is a mathematical expression used to predict the formation of a precipitate. It is calculated by multiplying the concentrations of the ions involved in the reaction. If Q is greater than the solubility product constant (Ksp), a precipitate will form.
Q = [A]^a[B]^b...
where [A] and [B] are the concentrations of the ions, and a and b are their respective stoichiometric coefficients.
Example
- Predict whether a precipitate will form when a solution of calcium chloride (CaCl2) is mixed with a solution of sodium carbonate (Na2CO3).
- Using the ion product expression, we can calculate Q: Q = [Ca^2+][CO3^2-]
- If Q is greater than Ksp, a precipitate of calcium carbonate will form.
Method 3: Solubility Product Constant (Ksp)

The solubility product constant (Ksp) is a mathematical expression used to predict the formation of a precipitate. It is calculated by multiplying the concentrations of the ions involved in the reaction. If Ksp is high, the compound is more soluble, while a low Ksp indicates a less soluble compound.
Ksp = [A]^a[B]^b...
where [A] and [B] are the concentrations of the ions, and a and b are their respective stoichiometric coefficients.
Example
- Predict whether a precipitate will form when a solution of copper(II) sulfate (CuSO4) is mixed with a solution of potassium hydroxide (KOH).
- Using the solubility product constant expression, we can calculate Ksp: Ksp = [Cu^2+][OH-]^2
- If Ksp is low, a precipitate of copper(II) hydroxide will form.
Method 4: Common Ion Effect

The common ion effect is a phenomenon where the presence of a common ion in a solution affects the solubility of a compound. If a solution contains a common ion, it can reduce the solubility of a compound, leading to precipitate formation.
Example
- Predict whether a precipitate will form when a solution of silver nitrate (AgNO3) is mixed with a solution of sodium chloride (NaCl).
- Using the common ion effect, we can see that the presence of chloride ions (Cl-) in the sodium chloride solution will reduce the solubility of silver chloride (AgCl), leading to precipitate formation.
Method 5: pH Effect

The pH effect is a phenomenon where the pH of a solution affects the solubility of a compound. Some compounds are more soluble at certain pH levels, while others are less soluble.
Example
- Predict whether a precipitate will form when a solution of aluminum sulfate (Al2(SO4)3) is mixed with a solution of sodium hydroxide (NaOH).
- Using the pH effect, we can see that the pH of the solution affects the solubility of aluminum hydroxide (Al(OH)3). At high pH levels, aluminum hydroxide is less soluble, leading to precipitate formation.
Method 6: Complex Ion Formation

Complex ion formation is a phenomenon where ions in a solution combine to form a complex ion. This can affect the solubility of a compound, leading to precipitate formation.
Example
- Predict whether a precipitate will form when a solution of copper(II) sulfate (CuSO4) is mixed with a solution of ammonia (NH3).
- Using complex ion formation, we can see that the copper(II) ion (Cu^2+) combines with ammonia (NH3) to form a complex ion (Cu(NH3)4^2+), reducing the concentration of copper(II) ions available for precipitate formation.
Method 7: Precipitate Formation in a Mixture of Solutions

Precipitate formation in a mixture of solutions can be predicted using the principles outlined above. By considering the concentrations of ions, solubility rules, ion product, and other factors, you can predict whether a precipitate will form when two or more solutions are mixed.
Example
- Predict whether a precipitate will form when a solution of calcium chloride (CaCl2) is mixed with a solution of sodium carbonate (Na2CO3) and a solution of copper(II) sulfate (CuSO4).
- Using the principles outlined above, we can predict that a precipitate of calcium carbonate (CaCO3) will form, while copper(II) ions will remain in solution.
Share Your Thoughts
Now that you have learned about the seven ways to predict precipitate formation, share your thoughts on how you can apply these methods in your own research or experiments. What do you think is the most important factor in predicting precipitate formation? Share your answers in the comments section below!
Frequently Asked Questions
What is precipitate formation?
+Precipitate formation is the process by which a solid forms from a solution. This occurs when a solution is supersaturated with a particular compound or when two solutions containing different ions are mixed.
What is the ion product (Q)?
+The ion product (Q) is a mathematical expression used to predict the formation of a precipitate. It is calculated by multiplying the concentrations of the ions involved in the reaction.
How does pH affect precipitate formation?
+pH can affect the solubility of a compound, leading to precipitate formation. Some compounds are more soluble at certain pH levels, while others are less soluble.