Water is one of the most unique and essential substances on our planet, and its properties are crucial for life as we know it. One of the key factors that make water so special is its incredible ability to form hydrogen bonds. In this article, we'll delve into the world of hydrogen bonding and explore just how many hydrogen bonds water can form.
Understanding Hydrogen Bonding

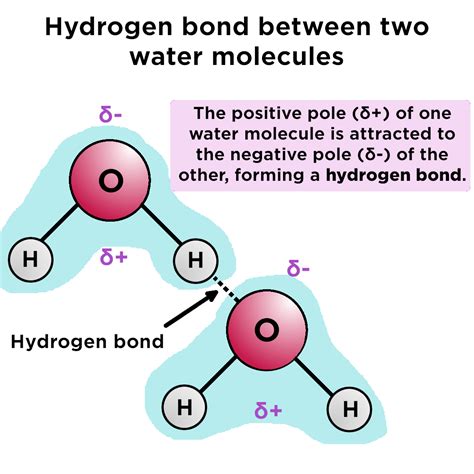
Hydrogen bonding is a type of intermolecular force that occurs between molecules with a hydrogen atom bonded to a highly electronegative atom, such as oxygen, nitrogen, or fluorine. This force is responsible for the unique properties of water, including its high boiling point, surface tension, and ability to dissolve a wide range of substances.
In the case of water, the oxygen atom is highly electronegative, which means it has a strong tendency to attract electrons towards itself. This creates a partial positive charge on the hydrogen atoms, allowing them to form weak bonds with the oxygen atoms of other water molecules. These bonds are known as hydrogen bonds.
Factors Affecting Hydrogen Bonding Capacity
The number of hydrogen bonds that water can form depends on several factors, including:
- Temperature: As temperature increases, the kinetic energy of the water molecules also increases, making it more difficult for them to form hydrogen bonds.
- Pressure: Increased pressure can force water molecules closer together, allowing them to form more hydrogen bonds.
- pH: Changes in pH can affect the availability of hydrogen ions, which can impact the number of hydrogen bonds that can form.
- Presence of other substances: Other substances, such as salts or sugars, can disrupt or enhance the formation of hydrogen bonds.
How Many Hydrogen Bonds Can Water Form?

Research suggests that a single water molecule can form up to four hydrogen bonds with other water molecules. This is because each water molecule has two hydrogen atoms and two lone pairs of electrons on the oxygen atom, allowing it to form two hydrogen bonds as a donor and two as an acceptor.
However, the actual number of hydrogen bonds that water can form in a given situation can vary significantly. In liquid water, the average number of hydrogen bonds per molecule is around 3.5-4.0. In ice, where the molecules are arranged in a crystalline structure, the number of hydrogen bonds per molecule can be as high as 4.5.
Implications of Hydrogen Bonding Capacity
The ability of water to form a large number of hydrogen bonds has significant implications for its properties and behavior. For example:
- High boiling point: The strong intermolecular forces between water molecules require a lot of energy to overcome, resulting in a high boiling point.
- Surface tension: The formation of hydrogen bonds between water molecules at the surface of a body of water creates a "skin" that allows it to resist external forces and maintain its shape.
- Solubility: The ability of water to form hydrogen bonds with other substances makes it an excellent solvent, allowing it to dissolve a wide range of salts, sugars, and other compounds.
Practical Applications of Hydrogen Bonding

The unique properties of water, including its high hydrogen bonding capacity, make it an essential component of many industrial and biological processes. Some examples include:
- Agriculture: Water's high surface tension and ability to form hydrogen bonds make it an ideal solvent for fertilizers and pesticides.
- Medicine: Hydrogen bonding plays a crucial role in the functioning of many biological molecules, including proteins and DNA.
- Chemical processing: Water's ability to dissolve a wide range of substances makes it a valuable solvent in many industrial processes.
Conclusion: Unlocking the Secrets of Hydrogen Bonding

In conclusion, the hydrogen bonding capacity of water is a fascinating topic that has significant implications for our understanding of its properties and behavior. By exploring the factors that affect hydrogen bonding and the practical applications of this phenomenon, we can gain a deeper appreciation for the importance of water in our daily lives.
We hope this article has provided you with a comprehensive understanding of the hydrogen bonding capacity of water. If you have any questions or comments, please don't hesitate to share them with us!
What is hydrogen bonding?
+Hydrogen bonding is a type of intermolecular force that occurs between molecules with a hydrogen atom bonded to a highly electronegative atom, such as oxygen, nitrogen, or fluorine.
How many hydrogen bonds can a single water molecule form?
+A single water molecule can form up to four hydrogen bonds with other water molecules.
What are some practical applications of hydrogen bonding?
+Hydrogen bonding plays a crucial role in many industrial and biological processes, including agriculture, medicine, and chemical processing.