Sulfur, a nonmetal element, plays a crucial role in the formation of covalent bonds in various compounds. Its ability to form covalent bonds is essential for its participation in numerous chemical reactions. The number of covalent bonds sulfur forms depends on the specific compound it is part of and the type of atoms it is bonding with.
What is Sulfur's Typical Valency?

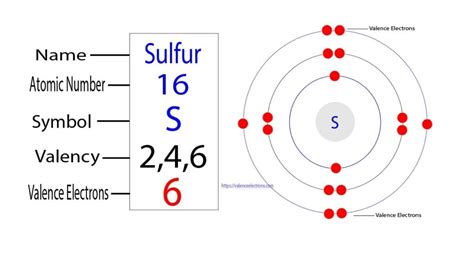
Sulfur typically exhibits a valency of 2, 4, or 6, depending on the compound it is part of. This means it can form two, four, or six covalent bonds with other atoms. In its most common form, sulfur exhibits a valency of 2, where it forms two covalent bonds with other atoms.
Sulfur's Covalent Bonds in Different Compounds
Sulfur's ability to form covalent bonds with various atoms is evident in its participation in different compounds. For instance:
- In hydrogen sulfide (H2S), sulfur forms two covalent bonds with hydrogen atoms.
- In sulfur dioxide (SO2), sulfur forms four covalent bonds with oxygen atoms.
- In sulfuric acid (H2SO4), sulfur forms six covalent bonds with oxygen and hydrogen atoms.
Why Does Sulfur Form Different Numbers of Covalent Bonds?

Sulfur's ability to form different numbers of covalent bonds can be attributed to its electronic configuration. Sulfur has six valence electrons, which it uses to form covalent bonds with other atoms. The number of covalent bonds sulfur forms depends on the number of electrons it shares with other atoms.
In general, sulfur tends to form covalent bonds with atoms that have a high electronegativity, such as oxygen and fluorine. This is because these atoms have a strong tendency to attract electrons, which allows sulfur to form multiple covalent bonds with them.
Factors Influencing Sulfur's Covalent Bonding
Several factors can influence sulfur's covalent bonding, including:
- Electronegativity: Atoms with high electronegativity tend to form more covalent bonds with sulfur.
- Atomic size: Larger atoms tend to form more covalent bonds with sulfur due to their increased electron-sharing capacity.
- Hybridization: Sulfur's ability to hybridize its orbitals allows it to form different numbers of covalent bonds.
Applications of Sulfur's Covalent Bonds

Sulfur's covalent bonds have numerous applications in various industries, including:
- Agriculture: Sulfur-containing compounds are used as fertilizers and pesticides.
- Pharmaceutical: Sulfur-containing compounds are used in the production of certain medications.
- Energy: Sulfur is used in the production of sulfuric acid, which is used in the manufacture of batteries and other energy storage devices.
Environmental Impact of Sulfur's Covalent Bonds
Sulfur's covalent bonds can have both positive and negative environmental impacts. For instance:
- Sulfur-containing compounds can contribute to air and water pollution.
- Sulfur is also essential for plant growth and is used in the production of fertilizers.
Conclusion: Sulfur's Covalent Bonds

In conclusion, sulfur's covalent bonds play a crucial role in its participation in various chemical reactions and compounds. Its ability to form different numbers of covalent bonds makes it an essential element in numerous industries. However, it is essential to consider the environmental impact of sulfur's covalent bonds to ensure sustainable use of this element.
We hope this article has provided you with a comprehensive understanding of sulfur's covalent bonds. Share your thoughts and comments below!
Take Action:
- Share this article with your friends and colleagues to spread awareness about sulfur's covalent bonds.
- Comment below with your thoughts on the importance of sulfur's covalent bonds in various industries.
- Explore more articles on chemistry and sulfur to deepen your understanding of this fascinating element.
What is sulfur's typical valency?
+Sulfur typically exhibits a valency of 2, 4, or 6, depending on the compound it is part of.
Why does sulfur form different numbers of covalent bonds?
+Sulfur's ability to form different numbers of covalent bonds can be attributed to its electronic configuration and the number of electrons it shares with other atoms.
What are the applications of sulfur's covalent bonds?
+Sulfur's covalent bonds have numerous applications in various industries, including agriculture, pharmaceutical, and energy.