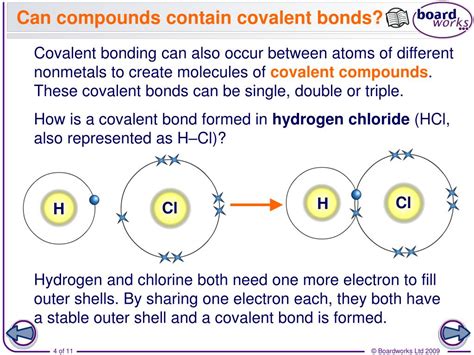
Hydrogen is the lightest and most abundant chemical element in the universe, and its unique properties make it a fundamental component of many molecules. One of the most important aspects of hydrogen is its covalent bonding capacity, which refers to its ability to form chemical bonds with other atoms. In this article, we will delve into the details of hydrogen's covalent bonding capacity, exploring how many bonds it can form and what factors influence its bonding behavior.

Hydrogen's Atomic Structure: The Key to Its Bonding Capacity
To understand hydrogen's covalent bonding capacity, we need to examine its atomic structure. Hydrogen has an atomic number of 1, which means it has one proton in its atomic nucleus. The atomic nucleus is surrounded by a single electron in the 1s orbital, which is the lowest energy level. This single electron is responsible for hydrogen's chemical reactivity and its ability to form covalent bonds.
How Many Covalent Bonds Can Hydrogen Form?
Hydrogen's covalent bonding capacity is determined by the number of electrons it can share with other atoms. In general, hydrogen can form one covalent bond with another atom, sharing its single electron to form a stable molecule. This is known as a sigma (σ) bond, which is a strong and stable chemical bond.
However, in some cases, hydrogen can form multiple covalent bonds with other atoms, a phenomenon known as hydrogen bonding. Hydrogen bonding occurs when a hydrogen atom bonded to a highly electronegative atom, such as oxygen or nitrogen, forms a weak bond with another electronegative atom. This type of bonding is weaker than a sigma bond but still plays a crucial role in the structure and properties of many molecules.
Factors Influencing Hydrogen's Covalent Bonding Capacity
Several factors can influence hydrogen's covalent bonding capacity, including:
- Electronegativity: The electronegativity of the atom bonded to hydrogen can affect its ability to form covalent bonds. Highly electronegative atoms, such as oxygen and nitrogen, can form stronger bonds with hydrogen.
- Atomic size: The size of the atom bonded to hydrogen can also influence its covalent bonding capacity. Smaller atoms, such as carbon and nitrogen, can form stronger bonds with hydrogen.
- Molecular environment: The molecular environment in which hydrogen is located can affect its covalent bonding capacity. For example, hydrogen bonding can occur in polar molecules, where the electronegative atom is bonded to a hydrogen atom.
Examples of Hydrogen's Covalent Bonding Capacity
Hydrogen's covalent bonding capacity is evident in many molecules, including:
- Water (H2O): In water, two hydrogen atoms form covalent bonds with a single oxygen atom, resulting in a stable molecule.
- Ammonia (NH3): In ammonia, three hydrogen atoms form covalent bonds with a single nitrogen atom, resulting in a stable molecule.
- Methane (CH4): In methane, four hydrogen atoms form covalent bonds with a single carbon atom, resulting in a stable molecule.

Applications of Hydrogen's Covalent Bonding Capacity
Hydrogen's covalent bonding capacity has many practical applications, including:
- Fuel cells: Hydrogen's ability to form covalent bonds with oxygen is the basis for fuel cell technology, which is used to generate electricity.
- Organic chemistry: Hydrogen's covalent bonding capacity is essential for the synthesis of many organic molecules, including drugs and plastics.
- Biological systems: Hydrogen bonding plays a crucial role in the structure and function of many biological molecules, including DNA and proteins.
Conclusion and Future Directions
In conclusion, hydrogen's covalent bonding capacity is a fundamental aspect of its chemistry, allowing it to form strong and stable bonds with other atoms. Understanding the factors that influence hydrogen's covalent bonding capacity is essential for predicting its behavior in different molecular environments.
As research continues to uncover the intricacies of hydrogen's covalent bonding capacity, we can expect to see new applications emerge in fields such as energy, medicine, and materials science.

We encourage you to share your thoughts on hydrogen's covalent bonding capacity and its applications in the comments section below. What do you think is the most significant impact of hydrogen's covalent bonding capacity on our daily lives?
What is the maximum number of covalent bonds that hydrogen can form?
+Hydrogen can form a maximum of one covalent bond with another atom, sharing its single electron to form a stable molecule.
What is hydrogen bonding, and how does it differ from covalent bonding?
+Hydrogen bonding is a type of weak bond that occurs between a hydrogen atom bonded to a highly electronegative atom and another electronegative atom. It is weaker than a covalent bond but still plays a crucial role in the structure and properties of many molecules.
What are some examples of molecules that exhibit hydrogen's covalent bonding capacity?
+Examples of molecules that exhibit hydrogen's covalent bonding capacity include water (H2O), ammonia (NH3), and methane (CH4).