Covalent bonds are a fundamental concept in chemistry, and understanding how they form and behave is crucial for understanding the structure and properties of molecules. One of the most important aspects of covalent bonds is the number of bonds that a carbon atom can form. In this article, we will delve into the world of covalent bonds and explore why carbon atoms typically form four covalent bonds.
Why Four Covalent Bonds Per Carbon Atom?

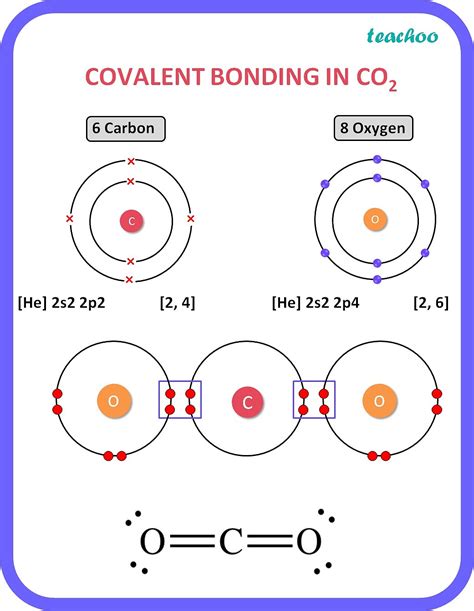
Carbon is a unique element in the periodic table, and its ability to form four covalent bonds is one of the key reasons why it is the basis of life on Earth. The reason why carbon atoms typically form four covalent bonds lies in its electronic configuration. Carbon has an atomic number of 6, which means it has six electrons in its outermost energy level. Of these six electrons, four are valence electrons, which are the electrons that participate in chemical bonding.
Electron Configuration and Covalent Bonding
The electron configuration of carbon is 1s² 2s² 2p², which means that the outermost energy level of carbon has two electrons in the s-orbital and two electrons in the p-orbitals. In order to achieve a stable electronic configuration, carbon atoms tend to form four covalent bonds, which allows them to have a full outer energy level.
This is because each covalent bond involves the sharing of a pair of electrons between two atoms. By forming four covalent bonds, carbon atoms can share four pairs of electrons, which fills the outer energy level and achieves a stable electronic configuration.
Types of Covalent Bonds Formed by Carbon

Carbon atoms can form different types of covalent bonds, including sigma (σ) bonds and pi (π) bonds. Sigma bonds are formed when two atoms share a pair of electrons in a head-on manner, resulting in a strong and stable bond. Pi bonds, on the other hand, are formed when two atoms share a pair of electrons in a side-by-side manner, resulting in a weaker and more reactive bond.
Carbon atoms can form four sigma bonds, which are typically stronger and more stable than pi bonds. This is why carbon atoms tend to form four covalent bonds, as it allows them to achieve a stable electronic configuration and form strong and stable molecules.
Examples of Molecules with Four Covalent Bonds Per Carbon Atom
Many molecules have four covalent bonds per carbon atom, including:
- Methane (CH₄): Each carbon atom in methane forms four covalent bonds with hydrogen atoms, resulting in a stable and unreactive molecule.
- Ethane (C₂H₆): Each carbon atom in ethane forms four covalent bonds, three with hydrogen atoms and one with another carbon atom.
- Propane (C₃H₈): Each carbon atom in propane forms four covalent bonds, three with hydrogen atoms and one with another carbon atom.
These molecules are all examples of saturated hydrocarbons, which are molecules that only contain carbon and hydrogen atoms and have only single bonds between the carbon atoms.
Exceptions to the Rule: Carbon Atoms with Fewer or More Covalent Bonds

While most carbon atoms form four covalent bonds, there are some exceptions to this rule. Some carbon atoms can form fewer or more covalent bonds, depending on the molecule they are part of.
For example, in molecules with double or triple bonds, carbon atoms can form fewer covalent bonds. In these molecules, the carbon atoms share more than one pair of electrons with each other, resulting in a stronger and more reactive bond.
On the other hand, some carbon atoms can form more covalent bonds, such as in molecules with five or six bonds. These molecules are typically highly reactive and unstable, and are not commonly found in nature.
Importance of Understanding Covalent Bonds
Understanding covalent bonds is crucial for understanding the structure and properties of molecules. By knowing how many covalent bonds a carbon atom can form, we can predict the shape and reactivity of a molecule.
This knowledge has many practical applications, including the development of new materials and medicines. For example, understanding the covalent bonds in a molecule can help us design new molecules with specific properties, such as higher strength or lower toxicity.
In conclusion, the ability of carbon atoms to form four covalent bonds is a fundamental aspect of chemistry, and understanding this concept is crucial for understanding the structure and properties of molecules. While there are some exceptions to this rule, the majority of carbon atoms form four covalent bonds, which allows them to achieve a stable electronic configuration and form strong and stable molecules.
We hope this article has helped you understand the importance of covalent bonds and why carbon atoms typically form four covalent bonds. If you have any questions or comments, please feel free to share them with us.
Why do carbon atoms typically form four covalent bonds?
+Carbon atoms typically form four covalent bonds because it allows them to achieve a stable electronic configuration. By sharing four pairs of electrons with other atoms, carbon atoms can fill their outer energy level and achieve a stable electronic configuration.
What are the different types of covalent bonds formed by carbon atoms?
+Carbon atoms can form two types of covalent bonds: sigma (σ) bonds and pi (π) bonds. Sigma bonds are formed when two atoms share a pair of electrons in a head-on manner, resulting in a strong and stable bond. Pi bonds, on the other hand, are formed when two atoms share a pair of electrons in a side-by-side manner, resulting in a weaker and more reactive bond.
What are some examples of molecules with four covalent bonds per carbon atom?
+Some examples of molecules with four covalent bonds per carbon atom include methane (CH₄), ethane (C₂H₆), and propane (C₃H₈). These molecules are all examples of saturated hydrocarbons, which are molecules that only contain carbon and hydrogen atoms and have only single bonds between the carbon atoms.