Chlorine, like other halogens, has a high tendency to form covalent bonds with other atoms. Understanding the bonding capacity of chlorine is essential in chemistry, as it helps in predicting the behavior of various compounds and molecules.
Chlorine is a member of the halogen family, which also includes fluorine, bromine, iodine, and astatine. These elements are located in Group 17 of the periodic table and have seven valence electrons in their outermost energy level. To achieve a stable noble gas configuration, halogens typically gain one electron to form a stable anion with a full outer energy level.
How Many Covalent Bonds Can Chlorine Form?

In general, chlorine can form one covalent bond with another atom. This is because chlorine has seven valence electrons, and when it shares one pair of electrons with another atom, it achieves a stable octet configuration. Chlorine's tendency to form a single covalent bond is due to its high electronegativity, which allows it to attract a shared pair of electrons towards itself.
However, under certain conditions, chlorine can form multiple covalent bonds with other atoms. This typically occurs when chlorine is bonded to highly electropositive atoms, such as hydrogen or certain metals. In these cases, chlorine can form multiple covalent bonds by sharing more than one pair of electrons with the other atom.
Examples of Chlorine's Bonding Capacity
- Hydrogen Chloride (HCl): In HCl, chlorine forms a single covalent bond with hydrogen by sharing one pair of electrons.
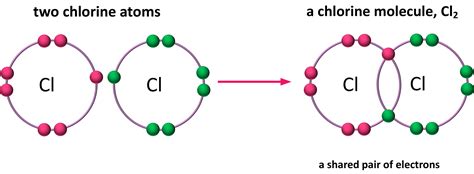
- Chlorine Gas (Cl2): In Cl2, two chlorine atoms form a single covalent bond by sharing one pair of electrons.
- Chlorine Oxides: In certain chlorine oxides, such as Cl2O or ClO2, chlorine forms multiple covalent bonds with oxygen atoms by sharing more than one pair of electrons.
Factors Affecting Chlorine's Bonding Capacity

Several factors can influence chlorine's bonding capacity, including:
- Electronegativity: Chlorine's high electronegativity allows it to attract shared electrons towards itself, making it more likely to form covalent bonds.
- Atomic size: Chlorine's relatively small atomic size allows it to form covalent bonds with other atoms more easily.
- Valence electrons: Chlorine's seven valence electrons make it highly reactive and prone to forming covalent bonds.
- Bonding partner: The nature of the atom bonded to chlorine can affect its bonding capacity. For example, highly electropositive atoms like hydrogen or metals can lead to multiple covalent bond formation.
Importance of Chlorine's Bonding Capacity

Understanding chlorine's bonding capacity is crucial in various fields, including:
- Chemical synthesis: Knowledge of chlorine's bonding capacity is essential in designing and synthesizing new compounds.
- Environmental science: Chlorine's reactivity and bonding capacity play a significant role in understanding environmental processes, such as water treatment and atmospheric chemistry.
- Materials science: Chlorine's bonding capacity is important in the development of new materials, such as polymers and ceramics.
Conclusion
Chlorine's bonding capacity is a fundamental aspect of its chemistry, and understanding it is essential in various fields. By recognizing the factors that influence chlorine's bonding capacity, we can better appreciate its reactivity and behavior in different compounds and molecules. Whether you're a student, researcher, or simply interested in chemistry, grasping chlorine's bonding capacity will help you navigate the fascinating world of chemical reactions and bonding.
We'd love to hear from you! Share your thoughts on chlorine's bonding capacity in the comments below.
What is the typical bonding capacity of chlorine?
+Chlorine typically forms one covalent bond with another atom.
What factors affect chlorine's bonding capacity?
+Electronegativity, atomic size, valence electrons, and bonding partner are some of the factors that affect chlorine's bonding capacity.
Why is understanding chlorine's bonding capacity important?
+Understanding chlorine's bonding capacity is crucial in chemical synthesis, environmental science, and materials science.