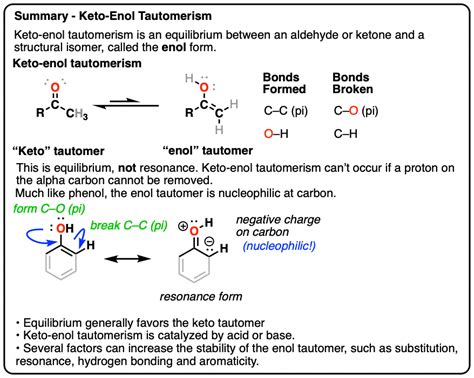
The keto tautomeric form is a fundamental concept in organic chemistry, playing a crucial role in understanding the structure and behavior of molecules. In this comprehensive guide, we will delve into the world of keto tautomeric forms, exploring their importance, mechanisms, and practical applications.
Understanding Keto Tautomeric Forms

In organic chemistry, tautomerism refers to the phenomenon where two or more molecules, differing only in the position of a hydrogen atom and a double bond, are in equilibrium with each other. Keto tautomeric forms are a specific type of tautomerism, where a keto group (a carbonyl group, C=O) is converted into an enol group (a hydroxyl group, OH, bonded to a carbon atom that is also bonded to a carbon-carbon double bond).
This conversion is made possible by the migration of a hydrogen atom from the alpha carbon atom (adjacent to the carbonyl group) to the oxygen atom of the carbonyl group. This process is reversible, meaning that the enol form can also convert back into the keto form.
Why are Keto Tautomeric Forms Important?
Keto tautomeric forms are essential in understanding various biological and chemical processes. For instance:
- Many biomolecules, such as sugars and amino acids, exhibit keto-enol tautomerism.
- Keto tautomeric forms play a crucial role in the mechanism of enzyme-catalyzed reactions.
- Understanding keto tautomeric forms is vital in the development of pharmaceuticals and other synthetic compounds.
Mechanisms of Keto Tautomeric Forms

The mechanism of keto tautomeric forms involves the following steps:
- Enolization: The keto form is converted into the enol form through the migration of a hydrogen atom from the alpha carbon atom to the oxygen atom of the carbonyl group.
- Protonation: The enol form is protonated, resulting in the formation of a resonance-stabilized enol ion.
- Deprotonation: The enol ion is deprotonated, leading to the formation of the keto form.
Factors Influencing Keto Tautomeric Forms
Several factors can influence the equilibrium between keto and enol forms, including:
- pH: Changes in pH can affect the equilibrium, with acidic conditions favoring the keto form and basic conditions favoring the enol form.
- Temperature: Increased temperature can shift the equilibrium towards the keto form.
- Solvent: The choice of solvent can influence the equilibrium, with polar solvents favoring the keto form and non-polar solvents favoring the enol form.
Practical Applications of Keto Tautomeric Forms

Keto tautomeric forms have numerous practical applications in various fields, including:
- Pharmaceuticals: Understanding keto tautomeric forms is crucial in the development of pharmaceuticals, as many drugs exhibit keto-enol tautomerism.
- Biochemistry: Keto tautomeric forms play a vital role in the mechanism of enzyme-catalyzed reactions.
- Materials Science: Keto tautomeric forms are used in the development of new materials, such as polymers and nanoparticles.
Examples of Keto Tautomeric Forms
Some examples of keto tautomeric forms include:
- Glucose: Glucose, a simple sugar, exhibits keto-enol tautomerism.
- Aspirin: Aspirin, a widely used pain reliever, exists in both keto and enol forms.
- Vitamin C: Vitamin C, an essential nutrient, exhibits keto-enol tautomerism.
Conclusion: Keto Tautomeric Forms in Perspective

In conclusion, keto tautomeric forms are a fundamental concept in organic chemistry, playing a crucial role in understanding the structure and behavior of molecules. By grasping the mechanisms and practical applications of keto tautomeric forms, researchers and scientists can unlock new insights into various biological and chemical processes.
We hope this comprehensive guide has provided you with a deeper understanding of keto tautomeric forms. Share your thoughts and questions in the comments below!
What is keto tautomeric form?
+Keto tautomeric form is a type of tautomerism where a keto group (a carbonyl group, C=O) is converted into an enol group (a hydroxyl group, OH, bonded to a carbon atom that is also bonded to a carbon-carbon double bond).
Why are keto tautomeric forms important?
+Keto tautomeric forms are essential in understanding various biological and chemical processes, including the mechanism of enzyme-catalyzed reactions and the development of pharmaceuticals.
What factors influence keto tautomeric forms?
+Factors such as pH, temperature, and solvent can influence the equilibrium between keto and enol forms.