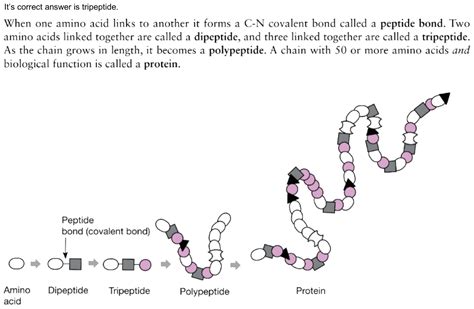
The process of forming tripeptides from dipeptides and amino acids is a fundamental aspect of biochemistry and peptide synthesis. Tripeptides are composed of three amino acids linked by peptide bonds, and understanding how to form them from smaller components is crucial for various applications in medicine, research, and biotechnology. In this article, we will explore three methods to form tripeptides from dipeptides and amino acids, highlighting the principles, mechanisms, and examples of each approach.
Method 1: Chemical Synthesis

Chemical synthesis is a widely used method for forming tripeptides from dipeptides and amino acids. This approach involves the use of chemical reagents and catalysts to facilitate the formation of peptide bonds between the dipeptide and the amino acid. One common method is the use of coupling reagents, such as dicyclohexylcarbodiimide (DCC) or 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC), which activate the carboxyl group of the dipeptide, allowing it to react with the amino group of the amino acid.
For example, to form the tripeptide Ala-Glu-Val from the dipeptide Ala-Glu and the amino acid Val, the following steps can be followed:
- Activate the carboxyl group of Ala-Glu using DCC or EDC.
- Add the amino acid Val to the reaction mixture.
- Allow the reaction to proceed for a specified time, typically several hours or overnight.
- Purify the resulting tripeptide using chromatography or other separation techniques.
Advantages and Limitations
Chemical synthesis offers several advantages, including:
- High yields and purity of the resulting tripeptide.
- Flexibility in terms of the choice of dipeptide and amino acid.
- Potential for large-scale production.
However, chemical synthesis also has some limitations:
- Requires specialized equipment and expertise.
- Can be time-consuming and labor-intensive.
- May result in the formation of unwanted side products.
Method 2: Enzymatic Synthesis

Enzymatic synthesis is an alternative approach to forming tripeptides from dipeptides and amino acids, utilizing enzymes as catalysts to facilitate the reaction. This method is based on the ability of certain enzymes, such as proteases or peptidases, to recognize and cleave specific peptide bonds.
One example of enzymatic synthesis is the use of the enzyme thermolysin to form the tripeptide Ala-Glu-Val from the dipeptide Ala-Glu and the amino acid Val. Thermolysin is a thermostable protease that can catalyze the formation of peptide bonds between amino acids.
- Mix the dipeptide Ala-Glu and the amino acid Val in a reaction buffer.
- Add thermolysin to the reaction mixture.
- Incubate the reaction at a suitable temperature, typically between 20-50°C.
- Monitor the reaction progress and purify the resulting tripeptide.
Advantages and Limitations
Enzymatic synthesis offers several advantages, including:
- Mild reaction conditions, reducing the risk of side reactions.
- High specificity and selectivity, resulting in high purity products.
- Potential for large-scale production.
However, enzymatic synthesis also has some limitations:
- Requires the availability of suitable enzymes.
- Can be sensitive to reaction conditions, such as temperature and pH.
- May result in lower yields compared to chemical synthesis.
Method 3: Solid-Phase Synthesis

Solid-phase synthesis is a technique used to form tripeptides from dipeptides and amino acids, where the dipeptide is attached to a solid support, such as a resin, and the amino acid is added in a stepwise manner.
One example of solid-phase synthesis is the use of the Fmoc (fluorenylmethyloxycarbonyl) strategy to form the tripeptide Ala-Glu-Val from the dipeptide Ala-Glu and the amino acid Val.
- Attach the dipeptide Ala-Glu to a solid support, such as a resin.
- Remove the Fmoc protecting group from the dipeptide.
- Add the amino acid Val to the reaction mixture.
- Allow the reaction to proceed for a specified time, typically several hours or overnight.
- Purify the resulting tripeptide using chromatography or other separation techniques.
Advantages and Limitations
Solid-phase synthesis offers several advantages, including:
- High yields and purity of the resulting tripeptide.
- Flexibility in terms of the choice of dipeptide and amino acid.
- Potential for large-scale production.
However, solid-phase synthesis also has some limitations:
- Requires specialized equipment and expertise.
- Can be time-consuming and labor-intensive.
- May result in the formation of unwanted side products.
In conclusion, the three methods described above offer distinct approaches to forming tripeptides from dipeptides and amino acids. Chemical synthesis, enzymatic synthesis, and solid-phase synthesis each have their advantages and limitations, and the choice of method depends on the specific requirements of the project, including the desired yield, purity, and scalability.
We hope this article has provided you with a comprehensive understanding of the different methods for forming tripeptides from dipeptides and amino acids. If you have any questions or comments, please feel free to share them below.
What is the difference between chemical synthesis and enzymatic synthesis?
+Chemical synthesis involves the use of chemical reagents and catalysts to facilitate the formation of peptide bonds, whereas enzymatic synthesis uses enzymes as catalysts to facilitate the reaction.
What is solid-phase synthesis, and how does it differ from other methods?
+Solid-phase synthesis involves attaching the dipeptide to a solid support and adding the amino acid in a stepwise manner. This method differs from other methods in that it allows for the formation of peptide bonds in a more controlled and efficient manner.
What are the advantages and limitations of each method?
+Each method has its advantages and limitations, including high yields and purity, flexibility in terms of the choice of dipeptide and amino acid, and potential for large-scale production. However, each method also has limitations, such as requiring specialized equipment and expertise, being time-consuming and labor-intensive, and resulting in the formation of unwanted side products.