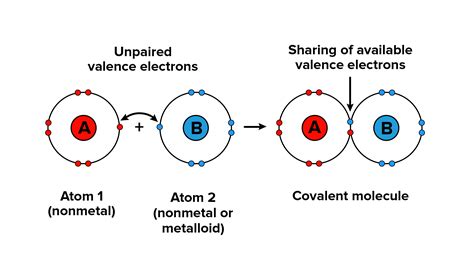
Covalent bonds are a fundamental concept in chemistry, allowing atoms to share electrons and form molecules. The process of covalent bond formation is crucial in understanding the structure and properties of matter. In this article, we will delve into the three primary ways covalent bonds form, exploring the mechanisms, benefits, and examples of each.
What are Covalent Bonds?

Covalent bonds are a type of chemical bond that involves the sharing of electron pairs between atoms. This sharing of electrons leads to the formation of a stable molecule, where the atoms are held together by the electrostatic attraction between the positively charged nuclei and the negatively charged electrons.
Way 1: Sigma (σ) Bond Formation

Sigma (σ) bonds are the most common type of covalent bond, formed when two atoms share a pair of electrons in a head-on overlap. This type of bond is typically strong and directional, resulting in a linear or trigonal planar molecular geometry.
Sigma bonds are formed through the overlap of atomic orbitals, where the orbitals of two atoms merge to form a single molecular orbital. This overlap can occur through end-to-end overlap, where the orbitals overlap along the bond axis, or side-by-side overlap, where the orbitals overlap perpendicular to the bond axis.
Examples of sigma bonds include:
- Hydrogen-hydrogen (H-H) bonds in hydrogen gas (H2)
- Carbon-carbon (C-C) bonds in ethane (C2H6)
- Oxygen-oxygen (O-O) bonds in oxygen gas (O2)
Benefits of Sigma Bonds
Sigma bonds have several benefits, including:
- High bond strength: Sigma bonds are typically strong and resistant to breaking.
- Directional bonding: Sigma bonds result in a specific molecular geometry, allowing for precise control over molecular shape.
- Versatility: Sigma bonds can form between a wide range of atoms, including hydrogen, carbon, oxygen, and nitrogen.
Way 2: Pi (π) Bond Formation

Pi (π) bonds are a type of covalent bond that forms when two atoms share a pair of electrons in a side-by-side overlap. This type of bond is typically weaker than sigma bonds and results in a more complex molecular geometry.
Pi bonds are formed through the overlap of atomic orbitals, where the orbitals of two atoms merge to form a single molecular orbital. This overlap occurs perpendicular to the bond axis, resulting in a nodal plane perpendicular to the bond axis.
Examples of pi bonds include:
- Carbon-carbon (C=C) bonds in ethene (C2H4)
- Nitrogen-nitrogen (N=N) bonds in nitrogen gas (N2)
- Oxygen-oxygen (O=O) bonds in ozone (O3)
Benefits of Pi Bonds
Pi bonds have several benefits, including:
- Multiple bonding: Pi bonds can form multiple bonds between atoms, resulting in increased bond strength and stability.
- Conjugation: Pi bonds can form conjugated systems, where the electrons are delocalized across multiple atoms, resulting in increased stability and reactivity.
- Aromaticity: Pi bonds can form aromatic systems, where the electrons are delocalized in a ring structure, resulting in increased stability and reactivity.
Way 3: Hydrogen Bonding

Hydrogen bonding is a type of covalent bond that forms when a hydrogen atom bonded to a highly electronegative atom, such as oxygen, nitrogen, or fluorine, is attracted to another electronegative atom. This type of bond is typically weak and results in a specific molecular geometry.
Hydrogen bonding is formed through the electrostatic attraction between the positively charged hydrogen atom and the negatively charged electronegative atom. This attraction results in a weak bond that can be broken easily.
Examples of hydrogen bonding include:
- Hydrogen bonds between water molecules (H2O)
- Hydrogen bonds between ammonia molecules (NH3)
- Hydrogen bonds between hydrogen fluoride molecules (HF)
Benefits of Hydrogen Bonding
Hydrogen bonding has several benefits, including:
- Solubility: Hydrogen bonding can increase the solubility of molecules in water and other polar solvents.
- Boiling point elevation: Hydrogen bonding can increase the boiling point of molecules, resulting in increased stability.
- Molecular recognition: Hydrogen bonding can facilitate molecular recognition, where molecules recognize and bind to specific targets.
As we have seen, covalent bonds can form through three primary mechanisms: sigma (σ) bond formation, pi (π) bond formation, and hydrogen bonding. Each of these mechanisms has its unique benefits and characteristics, resulting in a wide range of molecular structures and properties.
In conclusion, covalent bonds are a fundamental concept in chemistry, allowing atoms to share electrons and form molecules. Understanding the three primary ways covalent bonds form is crucial in understanding the structure and properties of matter. We hope this article has provided you with a comprehensive understanding of covalent bonds and their importance in chemistry.
What is the main difference between sigma and pi bonds?
+The main difference between sigma and pi bonds is the type of overlap between atomic orbitals. Sigma bonds form through end-to-end overlap, while pi bonds form through side-by-side overlap.
What is the purpose of hydrogen bonding?
+Hydrogen bonding is a type of covalent bond that forms between a hydrogen atom bonded to a highly electronegative atom and another electronegative atom. Its purpose is to facilitate molecular recognition and increase the solubility of molecules in water and other polar solvents.
What are some examples of pi bonds?
+Examples of pi bonds include carbon-carbon (C=C) bonds in ethene (C2H4), nitrogen-nitrogen (N=N) bonds in nitrogen gas (N2), and oxygen-oxygen (O=O) bonds in ozone (O3).