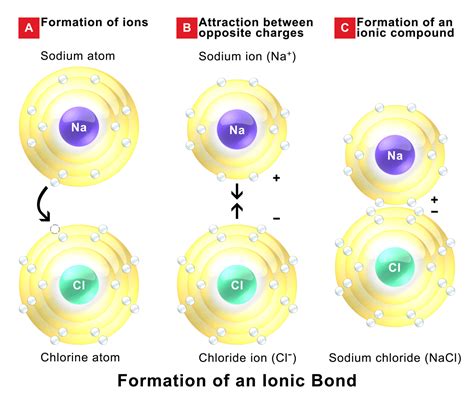
Nonmetals are elements that are located in the upper right corner of the periodic table, and they are typically unable to form ionic bonds with each other naturally. Ionic bonds are a type of chemical bond that involves the transfer of electrons between two atoms, resulting in the formation of ions with opposite charges. This type of bonding typically occurs between a metal and a nonmetal, where the metal loses one or more electrons to form a cation, and the nonmetal gains one or more electrons to form an anion.
However, there are some exceptions to this general rule. In certain cases, nonmetals can form ionic bonds with each other naturally, although this is relatively rare. This can occur when two nonmetals have a large difference in electronegativity, which is a measure of an atom's ability to attract electrons. When the difference in electronegativity is large enough, one nonmetal can pull electrons away from the other, resulting in the formation of ions with opposite charges.

For example, in the case of the compound hydrogen fluoride (HF), the difference in electronegativity between hydrogen and fluorine is large enough to result in the formation of ions with opposite charges. In this compound, the fluorine atom pulls an electron away from the hydrogen atom, resulting in the formation of a fluoride ion (F-) and a hydrogen ion (H+). The fluoride ion has a strong tendency to attract the hydrogen ion, resulting in the formation of a strong ionic bond.
Similarly, in the case of the compound ammonia (NH3), the difference in electronegativity between nitrogen and hydrogen is large enough to result in the formation of ions with opposite charges. In this compound, the nitrogen atom pulls electrons away from the hydrogen atoms, resulting in the formation of a nitrogen ion (N3-) and hydrogen ions (H+). The nitrogen ion has a strong tendency to attract the hydrogen ions, resulting in the formation of a strong ionic bond.
Why Don't Nonmetals Typically Form Ionic Bonds With Each Other?
Nonmetals do not typically form ionic bonds with each other because they tend to have similar electronegativities. Electronegativity is a measure of an atom's ability to attract electrons, and when two atoms have similar electronegativities, they are less likely to form ions with opposite charges. Instead, nonmetals tend to form covalent bonds with each other, which involve the sharing of electrons between atoms rather than the transfer of electrons.

For example, in the case of the compound oxygen (O2), the two oxygen atoms have similar electronegativities, and as a result, they form a covalent bond with each other. In this compound, the two oxygen atoms share a pair of electrons to form a strong covalent bond.
Similarly, in the case of the compound nitrogen (N2), the two nitrogen atoms have similar electronegativities, and as a result, they form a covalent bond with each other. In this compound, the two nitrogen atoms share a triple bond, which involves the sharing of three pairs of electrons.
Examples of Nonmetals That Can Form Ionic Bonds With Each Other
While nonmetals do not typically form ionic bonds with each other, there are some exceptions to this general rule. Here are a few examples of nonmetals that can form ionic bonds with each other:
- Hydrogen and fluorine: As mentioned earlier, the difference in electronegativity between hydrogen and fluorine is large enough to result in the formation of ions with opposite charges. In the compound hydrogen fluoride (HF), the fluorine atom pulls an electron away from the hydrogen atom, resulting in the formation of a fluoride ion (F-) and a hydrogen ion (H+).
- Nitrogen and oxygen: The difference in electronegativity between nitrogen and oxygen is large enough to result in the formation of ions with opposite charges. In the compound nitrogen oxide (NO), the oxygen atom pulls an electron away from the nitrogen atom, resulting in the formation of a nitrogen ion (N+) and an oxygen ion (O-).
- Carbon and fluorine: The difference in electronegativity between carbon and fluorine is large enough to result in the formation of ions with opposite charges. In the compound carbon tetrafluoride (CF4), the fluorine atoms pull electrons away from the carbon atom, resulting in the formation of a carbon ion (C4+) and fluorine ions (F-).

What Are the Conditions Necessary for Nonmetals to Form Ionic Bonds With Each Other?
For nonmetals to form ionic bonds with each other, the following conditions must be met:
- The difference in electronegativity between the two nonmetals must be large enough to result in the formation of ions with opposite charges.
- The two nonmetals must have a large difference in ionization energy, which is the energy required to remove an electron from an atom.
- The two nonmetals must have a large difference in electron affinity, which is the energy released when an electron is added to an atom.
When these conditions are met, the nonmetals can form ions with opposite charges, which are attracted to each other to form a strong ionic bond.
What Are the Properties of Ionic Bonds Formed Between Nonmetals?
Ionic bonds formed between nonmetals have several distinct properties, including:
- High melting and boiling points: Ionic bonds are typically strong and require a lot of energy to break, which results in high melting and boiling points.
- High solubility in water: Ionic compounds are typically highly soluble in water, which is due to the ability of the ions to interact with water molecules.
- Conductivity: Ionic compounds are typically good conductors of electricity, which is due to the ability of the ions to move freely.

What Are the Applications of Ionic Bonds Formed Between Nonmetals?
Ionic bonds formed between nonmetals have several important applications, including:
- Medicine: Ionic compounds are used in medicine to treat a variety of conditions, including high blood pressure and heart disease.
- Energy: Ionic compounds are used in energy storage and conversion applications, including batteries and fuel cells.
- Materials science: Ionic compounds are used in materials science to create new materials with unique properties, including high-temperature superconductors.
In conclusion, while nonmetals do not typically form ionic bonds with each other, there are some exceptions to this general rule. When the difference in electronegativity between two nonmetals is large enough, they can form ions with opposite charges, which are attracted to each other to form a strong ionic bond. These bonds have several distinct properties, including high melting and boiling points, high solubility in water, and conductivity. They also have several important applications, including medicine, energy, and materials science.
We hope this article has provided you with a better understanding of ionic bonds formed between nonmetals. If you have any questions or comments, please feel free to ask.
What is an ionic bond?
+An ionic bond is a type of chemical bond that involves the transfer of electrons between two atoms, resulting in the formation of ions with opposite charges.
What are the conditions necessary for nonmetals to form ionic bonds with each other?
+The conditions necessary for nonmetals to form ionic bonds with each other include a large difference in electronegativity, a large difference in ionization energy, and a large difference in electron affinity.
What are the properties of ionic bonds formed between nonmetals?
+The properties of ionic bonds formed between nonmetals include high melting and boiling points, high solubility in water, and conductivity.