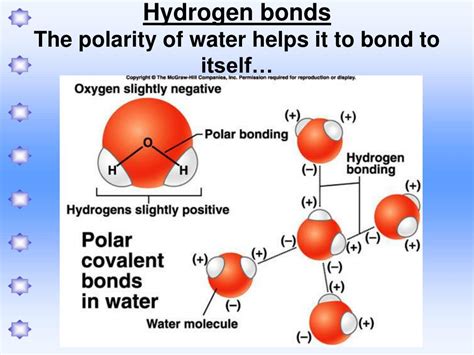
Polar molecules are a class of molecules that have a permanent electric dipole moment, meaning they have a slightly positive charge on one end and a slightly negative charge on the other end. This polarity allows them to interact with other molecules in a specific way, leading to the formation of various types of intermolecular forces. One of the most important types of intermolecular forces is the hydrogen bond, which plays a crucial role in many biological and chemical processes.
Hydrogen bonding is a type of intermolecular force that arises between a hydrogen atom bonded to a highly electronegative atom (such as oxygen, nitrogen, or fluorine) and another electronegative atom bearing a lone pair of electrons. This force is responsible for the high boiling and melting points of substances like water and ammonia, and is also essential for the structure and function of biological molecules like DNA and proteins.
But can polar molecules form hydrogen bonds? In this article, we will explore the answer to this question in detail.
What are Hydrogen Bonds?

Hydrogen bonds are a type of intermolecular force that arises between a hydrogen atom bonded to a highly electronegative atom (such as oxygen, nitrogen, or fluorine) and another electronegative atom bearing a lone pair of electrons. This force is responsible for the high boiling and melting points of substances like water and ammonia, and is also essential for the structure and function of biological molecules like DNA and proteins.
Hydrogen bonds are typically stronger than other types of intermolecular forces, such as van der Waals forces and dipole-dipole interactions. They are also highly directional, meaning that they only occur between specific atoms or groups of atoms.
Requirements for Hydrogen Bonding
For hydrogen bonding to occur, several requirements must be met:
- The molecule must have a hydrogen atom bonded to a highly electronegative atom (such as oxygen, nitrogen, or fluorine).
- The electronegative atom must have a lone pair of electrons.
- The molecule must be in close proximity to another molecule that has an electronegative atom with a lone pair of electrons.
Polar Molecules and Hydrogen Bonding

Polar molecules are a class of molecules that have a permanent electric dipole moment, meaning they have a slightly positive charge on one end and a slightly negative charge on the other end. This polarity allows them to interact with other molecules in a specific way, leading to the formation of various types of intermolecular forces.
Polar molecules can form hydrogen bonds with other molecules that meet the requirements for hydrogen bonding. However, not all polar molecules can form hydrogen bonds. For example, molecules that have a hydrogen atom bonded to a carbon atom (such as methane) are not able to form hydrogen bonds, because the carbon atom is not electronegative enough to create a significant dipole moment.
Examples of Polar Molecules that Can Form Hydrogen Bonds
Some examples of polar molecules that can form hydrogen bonds include:
- Water (H2O): Water molecules have a hydrogen atom bonded to an oxygen atom, which is highly electronegative. This allows water molecules to form hydrogen bonds with other water molecules.
- Ammonia (NH3): Ammonia molecules have a hydrogen atom bonded to a nitrogen atom, which is highly electronegative. This allows ammonia molecules to form hydrogen bonds with other ammonia molecules.
- Hydrogen fluoride (HF): Hydrogen fluoride molecules have a hydrogen atom bonded to a fluorine atom, which is highly electronegative. This allows hydrogen fluoride molecules to form hydrogen bonds with other hydrogen fluoride molecules.
Importance of Hydrogen Bonding in Polar Molecules

Hydrogen bonding is essential for the structure and function of many biological molecules, including DNA and proteins. It is also responsible for the high boiling and melting points of substances like water and ammonia.
In polar molecules, hydrogen bonding plays a crucial role in determining the physical and chemical properties of the substance. For example, the high boiling point of water is due to the strong hydrogen bonds between water molecules, which require a lot of energy to break.
Biological Importance of Hydrogen Bonding in Polar Molecules
Hydrogen bonding is essential for the structure and function of many biological molecules, including:
- DNA: Hydrogen bonding between DNA base pairs (adenine-thymine and guanine-cytosine) holds the double helix structure together.
- Proteins: Hydrogen bonding between amino acid residues helps to stabilize the 3D structure of proteins.
- Cell membranes: Hydrogen bonding between phospholipid molecules helps to maintain the structure and function of cell membranes.
Conclusion
In conclusion, polar molecules can form hydrogen bonds with other molecules that meet the requirements for hydrogen bonding. Hydrogen bonding is essential for the structure and function of many biological molecules, and plays a crucial role in determining the physical and chemical properties of substances like water and ammonia.
We hope this article has helped to clarify the relationship between polar molecules and hydrogen bonding. If you have any questions or comments, please feel free to share them with us.
What is the difference between a polar molecule and a nonpolar molecule?
+A polar molecule has a permanent electric dipole moment, meaning it has a slightly positive charge on one end and a slightly negative charge on the other end. A nonpolar molecule does not have a permanent electric dipole moment.
Can all polar molecules form hydrogen bonds?
+No, not all polar molecules can form hydrogen bonds. For hydrogen bonding to occur, the molecule must have a hydrogen atom bonded to a highly electronegative atom (such as oxygen, nitrogen, or fluorine), and the electronegative atom must have a lone pair of electrons.
What is the importance of hydrogen bonding in biological molecules?
+Hydrogen bonding is essential for the structure and function of many biological molecules, including DNA and proteins. It helps to stabilize the 3D structure of these molecules and is responsible for their physical and chemical properties.