Nitrogen is a fundamental element in the periodic table, known for its unique ability to form multiple bonds with other atoms. In its most common form, nitrogen is a diatomic molecule (N2), where two nitrogen atoms share a triple bond. However, under certain conditions, nitrogen can form four bonds with other atoms, which is a crucial aspect of its chemistry.
The ability of nitrogen to form four bonds is essential in various biological and industrial processes. For instance, nitrogen-based compounds are the backbone of life, forming the basis of amino acids, nucleotides, and other biomolecules. In agriculture, nitrogen-rich fertilizers are used to promote plant growth, while in industry, nitrogen-based compounds are used in the production of plastics, textiles, and pharmaceuticals.
In this article, we will explore three ways nitrogen forms four bonds, highlighting the underlying chemistry and providing examples of each.
1. Nitrogen Forms Four Bonds through Tetravalent Nitrogen Compounds

Tetravalent nitrogen compounds are a class of molecules where nitrogen is bonded to four other atoms. These compounds are formed when nitrogen is in a +4 oxidation state, meaning it has lost four electrons. Tetravalent nitrogen compounds are commonly found in organic chemistry, where they play a crucial role in the formation of complex molecules.
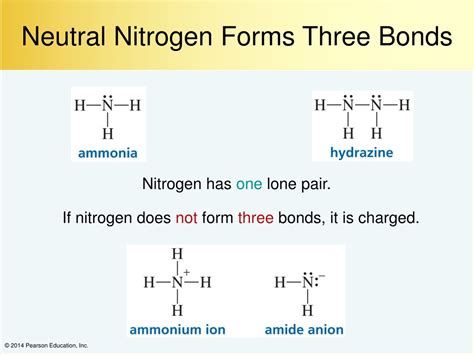
One example of a tetravalent nitrogen compound is ammonium (NH4+), which is formed when ammonia (NH3) reacts with an acid. In this compound, nitrogen is bonded to four hydrogen atoms, forming a stable ion. Another example is the nitrogen atom in the amino acid glutamine, where nitrogen is bonded to four carbon atoms.
Tetravalent nitrogen compounds are essential in various biological processes, including the synthesis of amino acids and the degradation of proteins. They are also used in industrial applications, such as the production of fertilizers and pharmaceuticals.
Formation of Tetravalent Nitrogen Compounds
Tetravalent nitrogen compounds are formed through various chemical reactions, including:
- Acid-base reactions: The reaction between ammonia and an acid forms the ammonium ion (NH4+).
- Oxidation reactions: The oxidation of ammonia or other nitrogen-containing compounds can form tetravalent nitrogen compounds.
- Alkylation reactions: The reaction between ammonia and alkyl halides can form tetravalent nitrogen compounds.
2. Nitrogen Forms Four Bonds through Nitrogen-Rich Anions

Nitrogen-rich anions are a class of molecules where nitrogen is bonded to multiple atoms, forming a negatively charged ion. These anions are formed when nitrogen is in a -3 or -4 oxidation state, meaning it has gained three or four electrons. Nitrogen-rich anions are commonly found in inorganic chemistry, where they play a crucial role in the formation of complex molecules.
One example of a nitrogen-rich anion is the azide ion (N3-), which is formed when nitrogen is bonded to three other nitrogen atoms. Another example is the nitride ion (N3-), which is formed when nitrogen is bonded to three other atoms, such as carbon or oxygen.
Nitrogen-rich anions are essential in various industrial applications, including the production of fertilizers, pharmaceuticals, and energetic materials. They are also used in biological processes, such as the synthesis of amino acids and the degradation of proteins.
Formation of Nitrogen-Rich Anions
Nitrogen-rich anions are formed through various chemical reactions, including:
- Reduction reactions: The reduction of nitrogen-containing compounds can form nitrogen-rich anions.
- Acid-base reactions: The reaction between nitrogen-containing compounds and bases can form nitrogen-rich anions.
- Alkylation reactions: The reaction between nitrogen-containing compounds and alkyl halides can form nitrogen-rich anions.
3. Nitrogen Forms Four Bonds through Metal-Nitrogen Complexes

Metal-nitrogen complexes are a class of molecules where nitrogen is bonded to a metal atom, forming a stable complex. These complexes are formed when nitrogen is in a +2 or +3 oxidation state, meaning it has lost two or three electrons. Metal-nitrogen complexes are commonly found in organometallic chemistry, where they play a crucial role in the formation of complex molecules.
One example of a metal-nitrogen complex is the iron-nitrogen complex, where nitrogen is bonded to an iron atom. Another example is the copper-nitrogen complex, where nitrogen is bonded to a copper atom.
Metal-nitrogen complexes are essential in various industrial applications, including the production of catalysts, pharmaceuticals, and materials. They are also used in biological processes, such as the synthesis of amino acids and the degradation of proteins.
Formation of Metal-Nitrogen Complexes
Metal-nitrogen complexes are formed through various chemical reactions, including:
- Coordination reactions: The reaction between nitrogen-containing compounds and metal atoms can form metal-nitrogen complexes.
- Oxidation reactions: The oxidation of nitrogen-containing compounds can form metal-nitrogen complexes.
- Alkylation reactions: The reaction between nitrogen-containing compounds and alkyl halides can form metal-nitrogen complexes.
In conclusion, nitrogen's ability to form four bonds is a crucial aspect of its chemistry, and it plays a vital role in various biological and industrial processes. The three ways nitrogen forms four bonds, through tetravalent nitrogen compounds, nitrogen-rich anions, and metal-nitrogen complexes, demonstrate the versatility of this element and its importance in the formation of complex molecules.
We invite you to share your thoughts on the importance of nitrogen in chemistry and its applications in various fields. How do you think nitrogen's ability to form four bonds contributes to its unique properties and uses? Share your comments below!
What is the oxidation state of nitrogen in tetravalent nitrogen compounds?
+The oxidation state of nitrogen in tetravalent nitrogen compounds is +4.
What is an example of a nitrogen-rich anion?
+The azide ion (N3-) is an example of a nitrogen-rich anion.
What is the importance of metal-nitrogen complexes in industry?
+Metal-nitrogen complexes are used in the production of catalysts, pharmaceuticals, and materials.