Hydrogen bonding is a fundamental concept in chemistry, and it's essential to understand how different molecules form these bonds. In this article, we'll delve into the world of hydrogen bonding, specifically focusing on how HF (hydrogen fluoride) forms hydrogen bonds.
Hydrogen bonding is a type of intermolecular force that arises between molecules with a hydrogen atom bonded to a highly electronegative atom, such as oxygen, nitrogen, or fluorine. This force is responsible for the unique properties of many substances, including water, which is essential for life on Earth.

Understanding HF Molecules
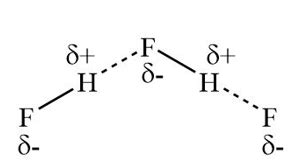
Before we dive into the world of hydrogen bonding, let's first understand the structure of HF molecules. HF is a diatomic molecule, consisting of one hydrogen atom bonded to one fluorine atom. The fluorine atom is highly electronegative, which means it has a strong tendency to attract electrons towards itself. This results in a partial positive charge on the hydrogen atom and a partial negative charge on the fluorine atom.
Polarity of HF Molecules
The polarity of HF molecules is crucial in understanding how they form hydrogen bonds. The partial positive charge on the hydrogen atom and the partial negative charge on the fluorine atom create a dipole moment, which is a measure of the molecule's polarity. This polarity allows HF molecules to interact with other molecules, including other HF molecules, through hydrogen bonding.
Three Ways HF Forms Hydrogen Bonds
Now that we've established the structure and polarity of HF molecules, let's explore the three ways HF forms hydrogen bonds.
1. Intermolecular Hydrogen Bonding
Intermolecular hydrogen bonding occurs between two or more HF molecules. In this type of bonding, the partial positive charge on the hydrogen atom of one HF molecule is attracted to the partial negative charge on the fluorine atom of another HF molecule. This attraction results in a weak intermolecular force that holds the molecules together.

2. Intramolecular Hydrogen Bonding
Intramolecular hydrogen bonding occurs within a single HF molecule. In this type of bonding, the partial positive charge on the hydrogen atom is attracted to the partial negative charge on the fluorine atom within the same molecule. This attraction results in a weak intramolecular force that stabilizes the molecule.
3. Hydrogen Bonding with Other Molecules
HF molecules can also form hydrogen bonds with other molecules that have a highly electronegative atom, such as oxygen or nitrogen. This type of bonding occurs when the partial positive charge on the hydrogen atom of the HF molecule is attracted to the partial negative charge on the electronegative atom of another molecule.

Importance of Hydrogen Bonding in HF
Hydrogen bonding plays a crucial role in the physical and chemical properties of HF. The unique properties of HF, such as its high boiling point and viscosity, can be attributed to the strong intermolecular hydrogen bonding between HF molecules.

Real-World Applications of HF Hydrogen Bonding
The unique properties of HF, resulting from its hydrogen bonding, have several real-world applications. For example, HF is used in the production of fluoropolymers, such as Teflon, which is used in non-stick cookware and other applications.

Conclusion: The Power of Hydrogen Bonding in HF
In conclusion, the unique properties of HF can be attributed to its hydrogen bonding. The three ways HF forms hydrogen bonds – intermolecular, intramolecular, and with other molecules – result in a molecule with unique physical and chemical properties. Understanding the importance of hydrogen bonding in HF can help us appreciate the complexity and beauty of molecular interactions.
We hope this article has provided you with a deeper understanding of the fascinating world of hydrogen bonding in HF. If you have any questions or comments, please feel free to share them below.
What is hydrogen bonding?
+Hydrogen bonding is a type of intermolecular force that arises between molecules with a hydrogen atom bonded to a highly electronegative atom, such as oxygen, nitrogen, or fluorine.
What is the polarity of HF molecules?
+The polarity of HF molecules arises from the partial positive charge on the hydrogen atom and the partial negative charge on the fluorine atom, resulting in a dipole moment.
What are some real-world applications of HF hydrogen bonding?
+HF is used in the production of fluoropolymers, such as Teflon, which is used in non-stick cookware and other applications.