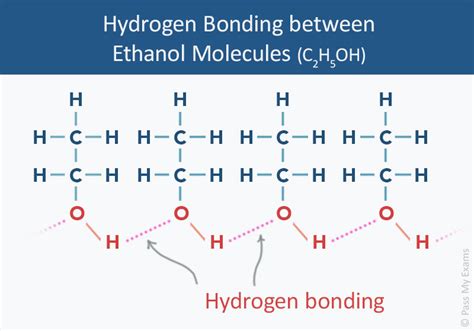
Ethanol, a common solvent and fuel, is a molecule that exhibits unique properties due to its ability to form hydrogen bonds. Hydrogen bonds are a type of intermolecular force that arises between molecules with a hydrogen atom bonded to a highly electronegative atom, such as oxygen, nitrogen, or fluorine. In the case of ethanol, the molecule has both a hydroxyl (-OH) group and a hydrocarbon chain, making it an ideal candidate for forming hydrogen bonds. In this article, we will explore the three ways ethanol forms hydrogen bonds and how these interactions impact its physical and chemical properties.
Hydrogen Bonding in Ethanol

Hydrogen bonding is a crucial aspect of ethanol's chemistry, and it plays a significant role in determining its physical properties, such as boiling point, viscosity, and solubility. Ethanol's ability to form hydrogen bonds is attributed to the presence of the hydroxyl (-OH) group, which is capable of acting as both a hydrogen bond donor and acceptor.
Mechanism of Hydrogen Bond Formation
The formation of hydrogen bonds in ethanol involves the interaction between the hydroxyl (-OH) group of one molecule and the oxygen atom of another molecule. This interaction is facilitated by the partial positive charge on the hydrogen atom and the partial negative charge on the oxygen atom. The resulting hydrogen bond is a weak electrostatic attraction that holds the molecules together.
Three Ways Ethanol Forms Hydrogen Bonds

Ethanol forms hydrogen bonds in three distinct ways:
1. Intermolecular Hydrogen Bonding
Intermolecular hydrogen bonding occurs between two or more ethanol molecules. This type of bonding is responsible for the physical properties of ethanol, such as its boiling point and viscosity. The hydroxyl (-OH) group of one molecule forms a hydrogen bond with the oxygen atom of another molecule, creating a network of weak electrostatic attractions that hold the molecules together.
2. Intramolecular Hydrogen Bonding
Intramolecular hydrogen bonding occurs within a single ethanol molecule. This type of bonding is responsible for the conformation of the molecule and plays a crucial role in determining its chemical properties. The hydroxyl (-OH) group forms a hydrogen bond with the oxygen atom of the same molecule, creating a stable conformation that influences the molecule's reactivity.
3. Hydrogen Bonding with Other Molecules
Ethanol also forms hydrogen bonds with other molecules, such as water and other polar solvents. This type of bonding is responsible for the solubility of ethanol in these solvents and plays a crucial role in determining its chemical properties. The hydroxyl (-OH) group of ethanol forms a hydrogen bond with the oxygen atom of the solvent molecule, creating a stable complex that facilitates dissolution.
Physical and Chemical Properties of Ethanol

The physical and chemical properties of ethanol are significantly influenced by its ability to form hydrogen bonds. Some of the key properties include:
- Boiling point: 78.3°C
- Viscosity: 1.2 cP (at 20°C)
- Solubility: soluble in water and other polar solvents
- Reactivity: reacts with strong acids and bases to form esters and ethers
The ability of ethanol to form hydrogen bonds also plays a crucial role in its biological properties, such as its toxicity and bioavailability.
Biological Properties of Ethanol

The biological properties of ethanol are significantly influenced by its ability to form hydrogen bonds. Some of the key properties include:
- Toxicity: ethanol is toxic to humans and animals in high concentrations
- Bioavailability: ethanol is readily absorbed by the body and distributed to tissues
- Metabolism: ethanol is metabolized by the liver and excreted in the urine
The ability of ethanol to form hydrogen bonds also plays a crucial role in its medicinal properties, such as its use as a solvent and an antiseptic.
Medicinal Properties of Ethanol

The medicinal properties of ethanol are significantly influenced by its ability to form hydrogen bonds. Some of the key properties include:
- Solvent properties: ethanol is used as a solvent in pharmaceutical preparations
- Antiseptic properties: ethanol is used as an antiseptic to disinfect wounds and surfaces
- Analgesic properties: ethanol is used as an analgesic to relieve pain
In conclusion, the ability of ethanol to form hydrogen bonds plays a crucial role in determining its physical, chemical, and biological properties. Understanding the mechanisms of hydrogen bond formation in ethanol is essential for appreciating its importance in various applications, from pharmaceuticals to biofuels.
We hope this article has provided valuable insights into the world of ethanol and its hydrogen bonding properties. If you have any questions or comments, please feel free to share them with us.
What is hydrogen bonding in ethanol?
+Hydrogen bonding in ethanol is a type of intermolecular force that arises between molecules with a hydrogen atom bonded to a highly electronegative atom, such as oxygen, nitrogen, or fluorine.
How does ethanol form hydrogen bonds?
+Ethanol forms hydrogen bonds in three distinct ways: intermolecular hydrogen bonding, intramolecular hydrogen bonding, and hydrogen bonding with other molecules.
What are the physical and chemical properties of ethanol?
+The physical and chemical properties of ethanol are significantly influenced by its ability to form hydrogen bonds, including its boiling point, viscosity, solubility, and reactivity.