The study of hydrogen bonding is a crucial aspect of understanding molecular interactions, particularly in the realm of organic chemistry. As a solvent, acetone is widely used in various chemical reactions and industrial applications. One question that often arises is whether acetone forms hydrogen bonds with other molecules. In this article, we will delve into the properties of acetone, the concept of hydrogen bonding, and the interactions between acetone and other molecules.

Understanding Hydrogen Bonding
Hydrogen bonding is a type of intermolecular force that arises between molecules with a hydrogen atom bonded to a highly electronegative atom, such as oxygen, nitrogen, or fluorine. This bonding is a result of the partial positive charge on the hydrogen atom being attracted to the partial negative charge on the electronegative atom of another molecule. Hydrogen bonds are relatively weak compared to covalent bonds but play a crucial role in the structure and properties of molecules.
Properties of Acetone
Acetone is a polar, aprotic solvent with the chemical formula (CH₃)₂CO. It has a molecular weight of 58.08 g/mol and a boiling point of 56.3°C. Acetone is widely used as a solvent in various industries, including pharmaceuticals, cosmetics, and chemical manufacturing. Its polar nature allows it to dissolve a wide range of substances, making it a versatile solvent.
Does Acetone Form Hydrogen Bonds?
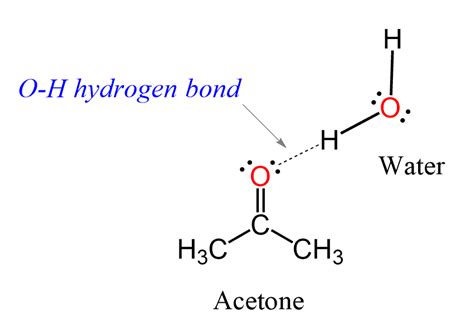
Acetone does not form hydrogen bonds with other molecules in the classical sense. As a ketone, acetone has a carbonyl group (C=O), which is polar but lacks a hydrogen atom bonded to a highly electronegative atom. However, acetone can participate in hydrogen bonding as a hydrogen bond acceptor.

In a mixture of acetone and a hydrogen bond donor, such as water or an alcohol, the oxygen atom of the carbonyl group in acetone can act as a hydrogen bond acceptor. This means that the oxygen atom can form a weak bond with the hydrogen atom of the hydrogen bond donor molecule.
Interactions between Acetone and Other Molecules
While acetone does not form hydrogen bonds in the classical sense, it can interact with other molecules through other intermolecular forces, such as dipole-dipole interactions and van der Waals forces. These interactions are important in understanding the behavior of acetone in mixtures and solutions.
- Dipole-dipole interactions: Acetone is a polar molecule, and as such, it can interact with other polar molecules through dipole-dipole interactions. This type of interaction occurs between the positive end of one dipole and the negative end of another dipole.
- Van der Waals forces: Acetone can also interact with other molecules through van der Waals forces, which are weak intermolecular forces that arise from the interaction between temporary dipoles.

Practical Applications of Acetone Interactions
Understanding the interactions between acetone and other molecules is crucial in various practical applications, such as:
- Solvent selection: Knowing the interactions between acetone and other molecules can help in selecting the most suitable solvent for a particular reaction or application.
- Reactions and synthesis: The interactions between acetone and other molecules can influence the outcome of reactions and synthesis.
- Separation and purification: The interactions between acetone and other molecules can be used to separate and purify substances.
Benefits of Understanding Acetone Interactions

Understanding the interactions between acetone and other molecules has several benefits, including:
- Improved solvent selection: Knowing the interactions between acetone and other molecules can help in selecting the most suitable solvent for a particular reaction or application.
- Enhanced reaction outcomes: Understanding the interactions between acetone and other molecules can influence the outcome of reactions and synthesis.
- Better separation and purification: The interactions between acetone and other molecules can be used to separate and purify substances.
Conclusion
In conclusion, while acetone does not form hydrogen bonds in the classical sense, it can participate in hydrogen bonding as a hydrogen bond acceptor. Additionally, acetone can interact with other molecules through other intermolecular forces, such as dipole-dipole interactions and van der Waals forces. Understanding these interactions is crucial in various practical applications, including solvent selection, reactions and synthesis, and separation and purification.
We hope this article has provided you with a comprehensive understanding of acetone interactions. If you have any questions or comments, please feel free to share them below.
What is the molecular weight of acetone?
+The molecular weight of acetone is 58.08 g/mol.
What is the boiling point of acetone?
+The boiling point of acetone is 56.3°C.
Can acetone form hydrogen bonds with other molecules?
+Acetone does not form hydrogen bonds in the classical sense, but it can participate in hydrogen bonding as a hydrogen bond acceptor.