Calcium and chlorine are two essential elements that play a significant role in various chemical reactions. Calcium, an alkaline earth metal, is widely used in construction, food, and pharmaceutical industries, while chlorine, a halogen, is commonly used as a disinfectant and sanitizer. When combined, calcium and chlorine can produce different compounds with distinct properties. In this article, we will explore three ways calcium reacts with chlorine.

Reaction 1: Formation of Calcium Chloride
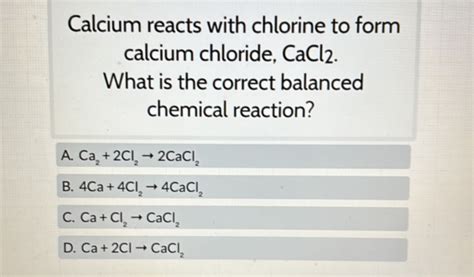
One of the most common reactions between calcium and chlorine is the formation of calcium chloride (CaCl2). This reaction occurs when calcium metal reacts with chlorine gas:
Ca (s) + Cl2 (g) → CaCl2 (s)
Calcium chloride is a white, crystalline solid that is highly soluble in water. It is widely used as a de-icer, a drying agent, and a flux in the steel industry. In addition, calcium chloride is used in food preservation, wastewater treatment, and as a coagulant in the production of tofu.

Industrial Applications of Calcium Chloride
Calcium chloride has various industrial applications, including:
- De-icing: Calcium chloride is used to lower the freezing point of water, making it an effective de-icer for roads, highways, and airport runways.
- Drying agent: Calcium chloride is used to remove moisture from gases and liquids, making it an essential component in the production of dry chemicals.
- Flux: Calcium chloride is used as a flux in the steel industry to remove impurities and improve the quality of steel.
Reaction 2: Formation of Calcium Hypochlorite
Another important reaction between calcium and chlorine is the formation of calcium hypochlorite (Ca(ClO)2). This reaction occurs when calcium hydroxide reacts with chlorine gas:
Ca(OH)2 (s) + Cl2 (g) → Ca(ClO)2 (s) + H2O (l)
Calcium hypochlorite is a white, crystalline solid that is highly soluble in water. It is widely used as a disinfectant and sanitizer in swimming pools, wastewater treatment, and food processing.

Uses of Calcium Hypochlorite
Calcium hypochlorite has various uses, including:
- Disinfectant: Calcium hypochlorite is used to disinfect swimming pools, wastewater, and surfaces.
- Sanitizer: Calcium hypochlorite is used to sanitize food processing equipment and utensils.
- Bleaching agent: Calcium hypochlorite is used as a bleaching agent in the textile and paper industries.
Reaction 3: Formation of Calcium Chlorate
The third reaction between calcium and chlorine is the formation of calcium chlorate (Ca(ClO3)2). This reaction occurs when calcium hydroxide reacts with chlorine dioxide:
Ca(OH)2 (s) + ClO2 (g) → Ca(ClO3)2 (s) + H2O (l)
Calcium chlorate is a white, crystalline solid that is highly soluble in water. It is widely used as an oxidizing agent in the production of explosives, matches, and fireworks.

Uses of Calcium Chlorate
Calcium chlorate has various uses, including:
- Oxidizing agent: Calcium chlorate is used as an oxidizing agent in the production of explosives, matches, and fireworks.
- Herbicide: Calcium chlorate is used as a herbicide to control weeds and grasses.
- Laboratory reagent: Calcium chlorate is used as a laboratory reagent in various analytical procedures.
In conclusion, the reactions between calcium and chlorine produce different compounds with distinct properties and uses. Understanding these reactions is essential for various industrial applications, including the production of chemicals, pharmaceuticals, and food products.

Now that you have learned about the three ways calcium reacts with chlorine, we encourage you to share your thoughts and questions in the comments section below. Do you have any experience working with calcium or chlorine compounds? Share your stories and expertise with us!
What is the most common reaction between calcium and chlorine?
+The most common reaction between calcium and chlorine is the formation of calcium chloride (CaCl2).
What is calcium hypochlorite used for?
+Calcium hypochlorite is used as a disinfectant and sanitizer in swimming pools, wastewater treatment, and food processing.
What is calcium chlorate used for?
+Calcium chlorate is used as an oxidizing agent in the production of explosives, matches, and fireworks.