Bromine, a reddish-brown, corrosive, and toxic chemical element, is a member of the halogen family. Its unique properties and electron configuration make it an essential element in various industries, including pharmaceuticals, water treatment, and oil refining. In this article, we will delve into the world of bromine and uncover its electron configuration in a simple and easy-to-understand manner.
As a chemical element, bromine's electron configuration is crucial in understanding its chemical behavior and properties. The electron configuration of an atom is a description of how electrons are arranged in its orbitals, which is essential in predicting its reactivity and interaction with other elements.
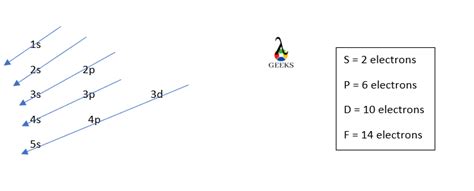
Before we dive into the electron configuration of bromine, let's first understand the basics of electron configuration. Electron configuration is a way of describing the arrangement of electrons in an atom, using a notation that shows the energy levels and orbitals occupied by electrons.

Understanding Electron Configuration Notation
Electron configuration notation is a shorthand way of describing the arrangement of electrons in an atom. It consists of a series of numbers and letters that represent the energy levels and orbitals occupied by electrons. The notation is written in a specific format, with the energy level number followed by the orbital type (s, p, d, or f) and the number of electrons in that orbital.
For example, the electron configuration of hydrogen is 1s1, which means that the single electron in hydrogen occupies the first energy level (1) and the s-orbital (s1).
Step-by-Step Guide to Bromine Electron Configuration
Now that we understand the basics of electron configuration notation, let's move on to the electron configuration of bromine. Bromine has an atomic number of 35, which means it has 35 electrons. To write the electron configuration of bromine, we need to follow a specific step-by-step process:
- Start by writing the energy level number, which is 1 for the first energy level, 2 for the second energy level, and so on.
- Next, write the orbital type (s, p, d, or f) followed by the number of electrons in that orbital.
Using this step-by-step process, we can write the electron configuration of bromine as follows:
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5
This notation tells us that bromine has:
- 2 electrons in the 1s orbital
- 2 electrons in the 2s orbital
- 6 electrons in the 2p orbital
- 2 electrons in the 3s orbital
- 6 electrons in the 3p orbital
- 2 electrons in the 4s orbital
- 10 electrons in the 3d orbital
- 5 electrons in the 4p orbital
Understanding the Bromine Electron Configuration
Now that we have written the electron configuration of bromine, let's take a closer look at what it means. The electron configuration of bromine tells us that it has a full outer energy level, with 7 electrons in the outermost energy level. This makes bromine a highly reactive element, as it is eager to gain or lose electrons to achieve a full outer energy level.
The electron configuration of bromine also tells us that it has a noble gas core, with a full inner energy level. This makes bromine a relatively stable element, as it is less reactive than other elements with partially filled inner energy levels.

Practical Applications of Bromine Electron Configuration
Understanding the electron configuration of bromine has several practical applications in various industries. For example:
- In the pharmaceutical industry, bromine is used as a disinfectant and antiseptic due to its highly reactive nature.
- In the water treatment industry, bromine is used as a disinfectant to kill bacteria and other microorganisms.
- In the oil refining industry, bromine is used as a catalyst to improve the efficiency of refining processes.
In conclusion, understanding the electron configuration of bromine is essential in understanding its chemical behavior and properties. By following a simple step-by-step process, we can write the electron configuration of bromine and gain insights into its reactivity and practical applications.
We hope this article has helped you uncover the bromine electron configuration in 1 easy step. Do you have any questions or comments about the article? Please feel free to share them below!
What is the atomic number of bromine?
+The atomic number of bromine is 35.
What is the electron configuration of bromine?
+The electron configuration of bromine is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5.
What are the practical applications of bromine electron configuration?
+The practical applications of bromine electron configuration include its use as a disinfectant and antiseptic in the pharmaceutical industry, as a disinfectant in the water treatment industry, and as a catalyst in the oil refining industry.