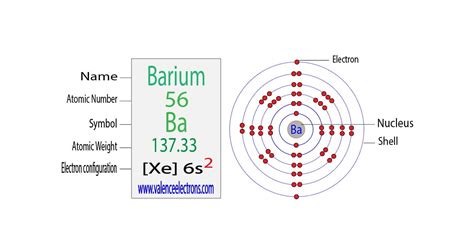
The fascinating world of chemistry, where the building blocks of matter are meticulously arranged to form the foundation of our universe. One crucial aspect of chemistry is understanding the electron configuration of elements, which plays a vital role in determining their properties and behavior. In this article, we will delve into the world of barium electron configuration, exploring its intricacies and significance.
The periodic table, a cornerstone of chemistry, is a tabular arrangement of elements, organized by their atomic number, electron configuration, and recurring chemical properties. Barium, a soft, silvery alkaline earth metal, is the fifth element in group 2 (formerly group IIa) of the periodic table. With an atomic number of 56, barium's electron configuration is a vital aspect of its chemical properties and behavior.

What is Electron Configuration?
Electron configuration is the arrangement of electrons in an atom, which is determined by the number of electrons in the atom and the energy levels or shells they occupy. The electron configuration of an element is a crucial factor in determining its chemical properties, reactivity, and bonding capabilities.
In an atom, electrons are arranged in a specific order, with each energy level or shell having a limited capacity. The electrons in an atom are arranged in the following order:
- The first energy level (1s orbital) can hold up to 2 electrons.
- The second energy level (2s and 2p orbitals) can hold up to 8 electrons.
- The third energy level (3s, 3p, and 3d orbitals) can hold up to 18 electrons.
- The fourth energy level (4s, 4p, 4d, and 4f orbitals) can hold up to 32 electrons.
Aufbau Principle and Hund's Rule
The Aufbau principle states that electrons fill the lowest available energy levels first. This principle is essential in determining the electron configuration of an element.
Hund's rule, on the other hand, states that when filling orbitals of equal energy, electrons occupy them singly and with parallel spins before pairing up. This rule is crucial in understanding the electron configuration of elements with multiple unpaired electrons.
Barium Electron Configuration
Now that we have a basic understanding of electron configuration, let's dive into the world of barium electron configuration.
Barium, with an atomic number of 56, has 56 electrons. The electron configuration of barium can be written as:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s² 4d¹⁰ 5p⁶ 6s²
This electron configuration indicates that barium has two electrons in the 1s orbital, two electrons in the 2s orbital, six electrons in the 2p orbital, and so on.

Valence Electrons
Valence electrons are the electrons in the outermost energy level of an atom, which participate in chemical bonding. In the case of barium, the valence electrons are the two electrons in the 6s orbital.
These valence electrons are responsible for the chemical properties of barium, including its reactivity and bonding capabilities.
Significance of Barium Electron Configuration
The electron configuration of barium plays a crucial role in its chemical properties and behavior. Some of the significant aspects of barium electron configuration include:
- Reactivity: Barium is highly reactive, due to its low ionization energy and high reactivity of its valence electrons.
- Bonding capabilities: Barium forms a variety of compounds, including oxides, sulfides, and halides, due to its ability to form ions with a +2 charge.
- Physical properties: Barium's electron configuration affects its physical properties, such as its melting and boiling points, density, and conductivity.

Applications of Barium Electron Configuration
The electron configuration of barium has numerous applications in various fields, including:
- Medical imaging: Barium sulfate is used as a contrast agent in medical imaging, due to its ability to absorb X-rays.
- Oil and gas drilling: Barium sulfate is used as a drilling fluid in oil and gas drilling, due to its high density and ability to suspend solids.
- Radiation shielding: Barium's high density and ability to absorb radiation make it an effective radiation shield.

Conclusion
In conclusion, the electron configuration of barium is a vital aspect of its chemical properties and behavior. Understanding the electron configuration of barium is essential for predicting its reactivity, bonding capabilities, and physical properties. The applications of barium electron configuration are numerous, ranging from medical imaging to radiation shielding.
We hope this article has provided you with a comprehensive understanding of barium electron configuration. If you have any questions or comments, please feel free to share them below.
What is the electron configuration of barium?
+The electron configuration of barium is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s² 4d¹⁰ 5p⁶ 6s².
What are the valence electrons of barium?
+The valence electrons of barium are the two electrons in the 6s orbital.
What are some applications of barium electron configuration?
+Some applications of barium electron configuration include medical imaging, oil and gas drilling, and radiation shielding.