Phosphorus is a highly reactive nonmetal element that plays a crucial role in various biological and chemical processes. One of the key characteristics of phosphorus is its ability to form ions, which are essential for its participation in numerous chemical reactions. In this article, we will explore the three primary ways phosphorus forms ions, highlighting their significance and applications.
Understanding Phosphorus Ions

Phosphorus ions are formed when phosphorus atoms gain or lose electrons to achieve a stable electronic configuration. This process is fundamental in chemistry, as it enables phosphorus to participate in various reactions and form compounds with other elements. Phosphorus ions can be either positively charged (cations) or negatively charged (anions), depending on the number of electrons gained or lost.
Electron Configuration of Phosphorus
To understand how phosphorus forms ions, it is essential to examine its electron configuration. Phosphorus has an atomic number of 15, which means it has 15 electrons in its atomic structure. The electron configuration of phosphorus is 1s² 2s² 2p⁶ 3s² 3p³, indicating that the outermost energy level (valence shell) has five electrons. This configuration makes phosphorus highly reactive, as it tends to gain or lose electrons to achieve a stable octet configuration.
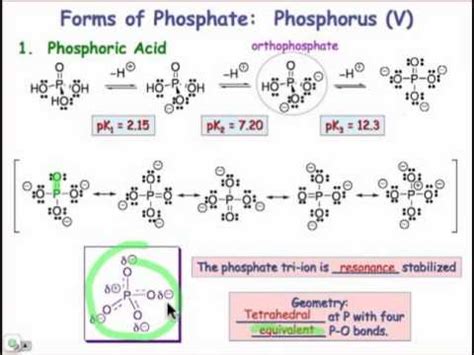
1. Formation of Phosphate Ions (PO43-)

Phosphate ions (PO43-) are one of the most common ions formed by phosphorus. This ion is formed when phosphorus reacts with oxygen to form phosphoric acid (H₃PO₄), which then dissociates into phosphate ions and hydrogen ions. The phosphate ion has a tetrahedral structure, with phosphorus at the center bonded to four oxygen atoms. Phosphate ions play a crucial role in various biological processes, including DNA and RNA synthesis, and are also used in fertilizers and detergents.
Importance of Phosphate Ions
Phosphate ions are essential for many biological processes, including:
- DNA and RNA synthesis: Phosphate ions are a critical component of nucleic acids, forming the backbone of DNA and RNA molecules.
- Energy transfer: Phosphate ions are involved in the transfer of energy in cells, particularly in the form of ATP (adenosine triphosphate).
- Fertilizers: Phosphate ions are used in fertilizers to promote plant growth and development.
2. Formation of Phosphite Ions (PO33-)

Phosphite ions (PO33-) are another type of ion formed by phosphorus. This ion is formed when phosphorus reacts with oxygen to form phosphorous acid (H₃PO₃), which then dissociates into phosphite ions and hydrogen ions. Phosphite ions have a trigonal pyramidal structure, with phosphorus at the center bonded to three oxygen atoms. Phosphite ions are used in various applications, including:
- Pesticides: Phosphite ions are used in pesticides to control fungal diseases in plants.
- Water treatment: Phosphite ions are used to remove heavy metals from water.
Applications of Phosphite Ions
Phosphite ions have various applications, including:
- Fungicides: Phosphite ions are used to control fungal diseases in plants, particularly in agriculture.
- Water treatment: Phosphite ions are used to remove heavy metals from water, making it safe for human consumption.
3. Formation of Phosphide Ions (P3-)

Phosphide ions (P3-) are a type of ion formed by phosphorus when it reacts with highly electropositive metals, such as sodium or potassium. This ion is formed when phosphorus gains three electrons to achieve a stable octet configuration. Phosphide ions are highly reactive and are used in various applications, including:
- Semiconductors: Phosphide ions are used in the production of semiconductors, which are essential for electronic devices.
- Catalysts: Phosphide ions are used as catalysts in various chemical reactions, particularly in the production of petrochemicals.
Importance of Phosphide Ions
Phosphide ions are essential for various industrial applications, including:
- Semiconductors: Phosphide ions are used in the production of semiconductors, which are critical for electronic devices.
- Catalysts: Phosphide ions are used as catalysts in various chemical reactions, particularly in the production of petrochemicals.
Now that you have read about the three ways phosphorus forms ions, we encourage you to share your thoughts and questions in the comments section below. How do you think phosphorus ions contribute to various biological and chemical processes? Do you have any experience working with phosphorus ions in a laboratory or industrial setting? Share your experiences and insights with us!
What is the electron configuration of phosphorus?
+The electron configuration of phosphorus is 1s² 2s² 2p⁶ 3s² 3p³.
What are phosphate ions used for?
+Phosphate ions are used in various biological processes, including DNA and RNA synthesis, energy transfer, and fertilizers.
What are phosphide ions used for?
+Phosphide ions are used in various industrial applications, including semiconductors and catalysts.