Chlorine is a highly reactive gas that is widely used in various industries, including water treatment, disinfection, and sanitation. As a halogen, chlorine readily forms ions when it comes into contact with other elements. In this article, we will explore the type of ion that chlorine forms and its properties.

What Ion Does Chlorine Form?
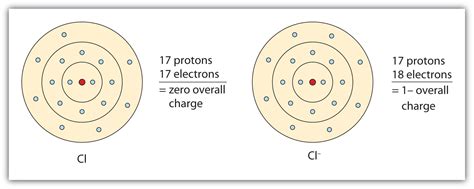
When chlorine reacts with other elements, it tends to gain an electron to form a negatively charged ion, known as a chloride ion (Cl-). This is because chlorine has a high electronegativity value, which means it has a strong tendency to attract electrons towards itself.
The chloride ion is a monatomic ion, meaning it consists of a single chlorine atom that has gained an electron. The chemical formula for the chloride ion is Cl-, and it has a charge of -1.
Properties of Chloride Ions
Chloride ions have several properties that make them important in various biological and industrial processes. Some of these properties include:
- High solubility: Chloride ions are highly soluble in water, which makes them useful in applications such as water treatment and sanitation.
- High reactivity: Chloride ions are highly reactive, which makes them useful in applications such as disinfection and bleaching.
- Conductivity: Chloride ions are good conductors of electricity, which makes them useful in applications such as electrochemistry.

Formation of Chloride Ions
Chloride ions can be formed through various chemical reactions, including:
- Reaction with metals: Chlorine can react with metals such as sodium and potassium to form chloride ions.
- Reaction with water: Chlorine can react with water to form hypochlorous acid, which can then dissociate into chloride ions.
- Reaction with other halogens: Chlorine can react with other halogens such as bromine and iodine to form chloride ions.
Examples of Chloride Ion Formation
Here are some examples of how chloride ions can be formed:
- 2Na (s) + Cl2 (g) → 2NaCl (s)
- Cl2 (g) + H2O (l) → HOCl (aq) + HCl (aq)
- Cl2 (g) + 2Br- (aq) → 2Cl- (aq) + Br2 (l)

Importance of Chloride Ions
Chloride ions play a crucial role in various biological and industrial processes, including:
- Water treatment: Chloride ions are used to disinfect and sanitize water supplies.
- Sanitation: Chloride ions are used to disinfect and sanitize surfaces and equipment.
- Electrochemistry: Chloride ions are used as an electrolyte in electrochemical reactions.
- Medicine: Chloride ions are used in various medical applications, including as a component of intravenous fluids.
Biological Importance of Chloride Ions
Chloride ions play a crucial role in various biological processes, including:
- Maintaining fluid balance: Chloride ions help to regulate the balance of fluids within the body.
- Maintaining blood pressure: Chloride ions help to regulate blood pressure by controlling the amount of fluid in the blood vessels.
- Regulating pH: Chloride ions help to regulate the pH of bodily fluids by controlling the amount of hydrogen ions.

Conclusion
In conclusion, chloride ions are an important type of ion that is formed when chlorine reacts with other elements. Chloride ions have several properties that make them useful in various biological and industrial processes, including high solubility, high reactivity, and conductivity. Chloride ions can be formed through various chemical reactions, including reaction with metals, water, and other halogens. Chloride ions play a crucial role in various biological and industrial processes, including water treatment, sanitation, electrochemistry, and medicine.
We hope this article has provided you with a comprehensive understanding of the type of ion that chlorine forms and its properties. If you have any further questions or comments, please feel free to ask.
What is the chemical formula for the chloride ion?
+The chemical formula for the chloride ion is Cl-.
What are some examples of how chloride ions can be formed?
+Chloride ions can be formed through various chemical reactions, including reaction with metals, water, and other halogens. Some examples include 2Na (s) + Cl2 (g) → 2NaCl (s), Cl2 (g) + H2O (l) → HOCl (aq) + HCl (aq), and Cl2 (g) + 2Br- (aq) → 2Cl- (aq) + Br2 (l).
What are some importance of chloride ions?
+Chloride ions play a crucial role in various biological and industrial processes, including water treatment, sanitation, electrochemistry, and medicine. Chloride ions are also important in various biological processes, including maintaining fluid balance, maintaining blood pressure, and regulating pH.