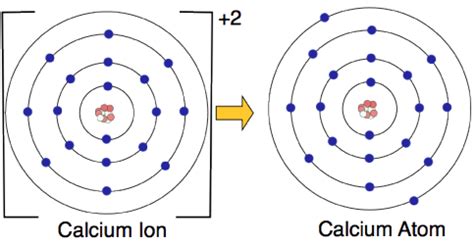
The Ion Calcium Forms: Understanding Their Importance and Impact

Calcium ions are a crucial component of various biological and chemical processes that occur in our bodies and the environment. These ions are formed when calcium atoms lose or gain electrons, resulting in the formation of charged particles. The ion calcium forms are essential for maintaining the balance of various bodily functions, from muscle contraction and nerve function to bone health and cellular metabolism.
The Significance of Ion Calcium Forms in Biological Processes
Ion calcium forms play a vital role in numerous biological processes, including:
- Muscle contraction and relaxation: Calcium ions help regulate muscle contraction and relaxation by binding to troponin and tropomyosin, allowing for the sliding of actin and myosin filaments.
- Nerve function: Calcium ions are involved in the transmission of nerve impulses, as they help regulate the release of neurotransmitters from nerve terminals.
- Bone health: Calcium ions are essential for bone mineralization, density, and strength.
- Cellular metabolism: Calcium ions participate in various cellular processes, such as glycolysis, gluconeogenesis, and lipid metabolism.
The Different Forms of Ion Calcium

Ion calcium can exist in various forms, each with distinct properties and functions. Some of the most common forms of ion calcium include:
-
Calcium ions (Ca2+)
+ Calcium ions are the most common form of ion calcium and are essential for various biological processes. + They have a +2 charge and are highly reactive. -
Calcium carbonate (CaCO3)
+ Calcium carbonate is a stable compound formed when calcium ions react with carbonate ions. + It is commonly found in rocks, shells, and skeletons. -
Calcium phosphate (Ca3(PO4)2)
+ Calcium phosphate is a compound formed when calcium ions react with phosphate ions. + It is essential for bone mineralization and density. -
Calcium chloride (CaCl2)
+ Calcium chloride is a compound formed when calcium ions react with chloride ions. + It is commonly used as a food additive and in various industrial applications.
The Importance of Ion Calcium Forms in Industrial Applications

Ion calcium forms have various industrial applications, including:
-
Food industry
+ Calcium ions are used as a food additive to enhance flavor, texture, and nutritional value. + Calcium carbonate is used as an anti-caking agent in food products. -
Pharmaceutical industry
+ Calcium ions are used in various pharmaceutical applications, including the production of calcium supplements and antacids. + Calcium carbonate is used as a filler in tablets and capsules. -
Construction industry
+ Calcium carbonate is used in the production of cement, concrete, and mortar. + Calcium ions are used in the manufacture of gypsum and plaster.
The Role of Ion Calcium Forms in Environmental Processes

Ion calcium forms play a significant role in various environmental processes, including:
-
Water treatment
+ Calcium ions are used in water treatment processes to remove impurities and soften water. + Calcium carbonate is used to neutralize acidic water. -
+ Calcium ions are used to remediate contaminated soil by binding to heavy metals and reducing their toxicity. + Calcium carbonate is used to neutralize acidic soil.
-
Atmospheric processes
+ Calcium ions are involved in atmospheric processes, including the formation of clouds and precipitation. + Calcium carbonate is used to reduce atmospheric carbon dioxide levels.
Practical Applications of Ion Calcium Forms

Ion calcium forms have various practical applications, including:
-
Medicine
+ Calcium ions are used in medical treatments, including the treatment of osteoporosis and hypocalcemia. + Calcium carbonate is used as an antacid to neutralize stomach acid. -
+ Calcium ions are used in agricultural applications, including the production of fertilizers and soil conditioners. + Calcium carbonate is used to neutralize acidic soil and improve crop yields.
-
+ Calcium ions are used in food production, including the manufacture of dairy products and beverages. + Calcium carbonate is used as a food additive to enhance flavor and texture.
Ion Calcium Forms in Research and Development

Ion calcium forms are being researched and developed for various applications, including:
-
Biomedical applications
+ Researchers are developing new biomedical applications for ion calcium forms, including the treatment of diseases and injuries. + Calcium ions are being investigated for their potential to enhance bone regeneration and repair. - <h3.Environmental remediation
- Researchers are developing new environmental remediation technologies that utilize ion calcium forms to clean up contaminated soil and water.
- Calcium ions are being investigated for their potential to remove heavy metals and other pollutants from the environment.
-
Industrial applications
+ Researchers are developing new industrial applications for ion calcium forms, including the production of novel materials and chemicals. + Calcium ions are being investigated for their potential to enhance the efficiency and sustainability of various industrial processes.
Conclusion and Future Directions
Ion calcium forms play a vital role in various biological, industrial, and environmental processes. Understanding the importance and impact of these forms is essential for advancing our knowledge and developing new applications. As research and development continue to uncover the potential of ion calcium forms, we can expect to see new and innovative applications emerge in the future.
We invite you to share your thoughts and questions on the topic of ion calcium forms in the comments section below. How do you think ion calcium forms will impact various industries and applications in the future?
What is the most common form of ion calcium?
+The most common form of ion calcium is calcium ions (Ca2+).
What are some practical applications of ion calcium forms?
+Ion calcium forms have various practical applications, including medicine, agriculture, food production, and industrial processes.
What is the role of ion calcium forms in environmental processes?
+Ions calcium forms play a significant role in various environmental processes, including water treatment, soil remediation, and atmospheric processes.