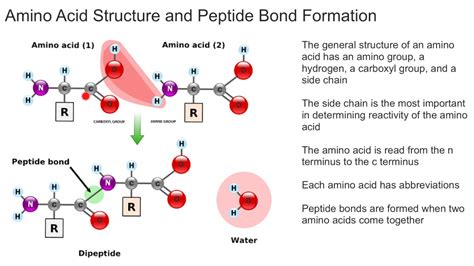
The building blocks of life, amino acids, are the fundamental components of proteins. These proteins are crucial for various biological processes, and their structure and function are determined by the types of bonds formed between amino acids. Understanding the different types of bonds is essential to grasp the complexity of protein structure and function.
Proteins are composed of amino acids linked together by peptide bonds, which are a type of covalent bond. However, this is not the only type of bond that forms between amino acids. In this article, we will explore the four main types of bonds that are formed between amino acids, including peptide bonds, hydrogen bonds, ionic bonds, and disulfide bridges.
Peptide Bonds

Peptide bonds are the primary covalent bonds that link amino acids together to form a polypeptide chain. These bonds are formed through a dehydration reaction, where the carboxyl group of one amino acid reacts with the amino group of another amino acid, resulting in the release of a water molecule. This reaction is catalyzed by enzymes and is a crucial step in protein synthesis.
The peptide bond is a strong covalent bond that provides stability to the protein structure. The bond is formed between the alpha-amino group of one amino acid and the alpha-carboxyl group of another amino acid. The resulting bond is a planar, rigid structure that provides a framework for the protein's secondary, tertiary, and quaternary structures.
Characteristics of Peptide Bonds
- Covalent bond
- Formed through dehydration reaction
- Links amino acids together to form a polypeptide chain
- Provides stability to protein structure
- Planar, rigid structure
Hydrogen Bonds

Hydrogen bonds are weak electrostatic interactions between the positively charged amino group and the negatively charged carboxyl group of adjacent amino acids. These bonds are crucial for the formation of protein secondary structures, such as alpha-helices and beta-sheets.
Hydrogen bonds are formed when the slightly positive hydrogen atom of an amino group is attracted to the slightly negative oxygen atom of a carboxyl group. This attraction is weak compared to covalent bonds, but it plays a significant role in protein structure and stability.
Characteristics of Hydrogen Bonds
- Weak electrostatic interaction
- Forms between positively charged amino group and negatively charged carboxyl group
- Crucial for protein secondary structure formation
- Weak compared to covalent bonds
Ionic Bonds

Ionic bonds, also known as salt bridges, are electrostatic interactions between positively charged amino groups and negatively charged carboxyl groups of adjacent amino acids. These bonds are stronger than hydrogen bonds and play a crucial role in protein tertiary structure formation.
Ionic bonds are formed when the positively charged amino group of one amino acid is attracted to the negatively charged carboxyl group of another amino acid. This attraction is stronger than hydrogen bonds and provides additional stability to the protein structure.
Characteristics of Ionic Bonds
- Electrostatic interaction
- Forms between positively charged amino group and negatively charged carboxyl group
- Stronger than hydrogen bonds
- Crucial for protein tertiary structure formation
Disulfide Bridges

Disulfide bridges are covalent bonds formed between the sulfur atoms of two cysteine amino acids. These bonds are crucial for protein tertiary and quaternary structure formation and provide additional stability to the protein structure.
Disulfide bridges are formed when the sulfur atoms of two cysteine amino acids are oxidized, resulting in the formation of a covalent bond. This bond is strong and provides stability to the protein structure.
Characteristics of Disulfide Bridges
- Covalent bond
- Forms between sulfur atoms of two cysteine amino acids
- Crucial for protein tertiary and quaternary structure formation
- Provides additional stability to protein structure
In conclusion, the four types of bonds formed between amino acids are crucial for protein structure and function. Peptide bonds provide a framework for protein structure, while hydrogen bonds, ionic bonds, and disulfide bridges provide additional stability and play a crucial role in protein secondary, tertiary, and quaternary structure formation. Understanding these bonds is essential for grasping the complexity of protein structure and function.
What is the primary covalent bond that links amino acids together?
+Peptide bonds are the primary covalent bonds that link amino acids together to form a polypeptide chain.
What type of bond is formed between the positively charged amino group and the negatively charged carboxyl group of adjacent amino acids?
+Ionic bonds, also known as salt bridges, are electrostatic interactions between positively charged amino groups and negatively charged carboxyl groups of adjacent amino acids.
What is the function of disulfide bridges in protein structure?
+Disulfide bridges provide additional stability to protein structure and play a crucial role in protein tertiary and quaternary structure formation.