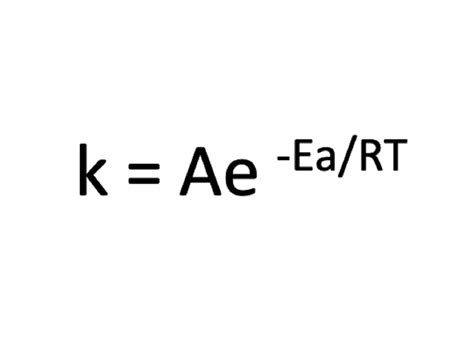Understanding the Arrhenius equation is crucial for anyone studying chemistry, particularly in the context of kinetics and reaction rates. This equation, developed by Svante Arrhenius in 1889, provides a fundamental relationship between the rate constant of a reaction and the temperature at which it occurs. The Arrhenius equation is a cornerstone in understanding how reactions are influenced by temperature, making it a critical component of chemical kinetics. In this article, we'll delve into two comprehensive ways to understand the Arrhenius equation, providing insights into its application, significance, and the underlying principles that make it a powerful tool in chemistry.

Understanding the Basics of the Arrhenius Equation
The Arrhenius equation is expressed as k = Ae^(-Ea/RT), where:
- k is the rate constant of the reaction.
- A is a pre-exponential factor, often related to the frequency of collisions.
- Ea is the activation energy, which is the energy barrier that must be overcome for the reaction to proceed.
- R is the gas constant.
- T is the temperature in Kelvin.

Understanding the components of the Arrhenius equation is the first step in appreciating its significance. The equation essentially tells us that the rate constant (k) of a reaction is influenced by two main factors: the frequency of collisions (A) and the energy barrier (Ea) that reactants must overcome. The role of temperature (T) is also critical, as it affects the energy of the reactants and thus the likelihood of successful collisions.
Breaking Down the Components
-
Activation Energy (Ea): This is the minimum amount of energy that the reactants must have for the reaction to occur. The value of Ea is specific to each reaction and is influenced by the nature of the reactants and the conditions under which the reaction takes place.
-
Pre-exponential Factor (A): This factor is related to the frequency of collisions and the orientation of the reactants during collisions. A higher value of A indicates a higher frequency of effective collisions, which can lead to a higher reaction rate.
-
Temperature (T): The temperature affects the kinetic energy of the reactants. At higher temperatures, the reactants have more energy, making it easier for them to overcome the activation energy barrier and react.
Detailed Analysis and Application of the Arrhenius Equation
Beyond understanding the basic components and their roles, a detailed analysis of the Arrhenius equation reveals its power in predicting and analyzing reaction rates. The equation is widely used in various fields, including chemistry, chemical engineering, and materials science, to study the kinetics of reactions and understand how temperature influences reaction rates.

Linearizing the Arrhenius Equation
One of the practical applications of the Arrhenius equation is its linearization, which allows for the easy extraction of activation energy (Ea) and the pre-exponential factor (A) from experimental data. By rearranging the equation into a linear form, ln(k) = ln(A) - (Ea/R)(1/T), and plotting ln(k) against 1/T, one can obtain a straight line with a slope of -Ea/R and an intercept of ln(A).
Catalysis and the Arrhenius Equation
Catalysts are substances that speed up chemical reactions without being consumed in the process. According to the Arrhenius equation, catalysts work by lowering the activation energy (Ea) required for the reaction to proceed, thereby increasing the rate constant (k) at a given temperature. This is crucial in industrial processes, where catalysts are used to enhance reaction rates, selectivity, and efficiency.
Limitations and Assumptions of the Arrhenius Equation
While the Arrhenius equation is a powerful tool for understanding reaction kinetics, it has its limitations. It assumes that the reaction rate is controlled by a single step, which might not always be the case. Additionally, the equation does not account for the effects of pressure, solvent, or other reactants on the reaction rate. Therefore, its application should be considered in the context of the specific reaction and conditions being studied.
In conclusion, understanding the Arrhenius equation is essential for anyone delving into the world of chemical kinetics. By grasping the equation's components, significance, and applications, one can gain a deeper insight into how reactions are influenced by temperature and how this knowledge can be harnessed in various fields. Whether you're a student, researcher, or professional in chemistry, the Arrhenius equation is a fundamental concept that will undoubtedly play a significant role in your understanding of chemical reactions.

Let's continue the discussion in the comments section. Share your thoughts on the Arrhenius equation and its applications. How do you think understanding this equation can benefit your studies or professional endeavors? Share your experiences or questions, and let's keep the conversation going!
What is the main application of the Arrhenius equation?
+The main application of the Arrhenius equation is to understand how the rate of a chemical reaction is influenced by temperature. It provides a relationship between the rate constant of a reaction and the temperature at which it occurs.
What are the limitations of the Arrhenius equation?
+The Arrhenius equation assumes that the reaction rate is controlled by a single step and does not account for the effects of pressure, solvent, or other reactants on the reaction rate. Its application should be considered in the context of the specific reaction and conditions being studied.
How does a catalyst affect the Arrhenius equation?
+A catalyst lowers the activation energy (Ea) required for the reaction to proceed, thereby increasing the rate constant (k) at a given temperature, according to the Arrhenius equation.