Carbon dioxide, a byproduct of cellular metabolism, is transported from tissues to the lungs in the blood. While it may seem like a simple process, the transportation of CO2 is a complex mechanism that involves multiple steps and various forms of the gas. One of the primary ways CO2 is transported in the blood is in the form of bicarbonate.
Why is CO2 Transport Important?

Carbon dioxide is a waste product of cellular respiration, the process by which cells generate energy from glucose. When CO2 builds up in tissues, it can lead to acidosis, a condition in which the blood becomes too acidic. To prevent this, the body has developed a system to transport CO2 from tissues to the lungs, where it can be exhaled.
How is CO2 Transported in the Blood?
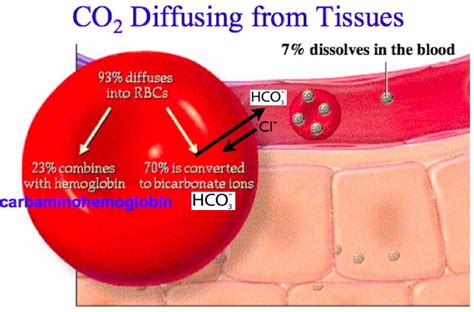
There are three primary ways CO2 is transported in the blood:
- Dissolved in plasma: A small amount of CO2 dissolves in the plasma, the liquid portion of the blood.
- Bound to hemoglobin: CO2 binds to hemoglobin, the protein in red blood cells that carries oxygen.
- Converted to bicarbonate: CO2 reacts with water to form carbonic acid, which then dissociates into bicarbonate ions and hydrogen ions.
The Role of Bicarbonate in CO2 Transport

Bicarbonate is the primary form of CO2 transport in the blood. When CO2 enters the blood, it reacts with water to form carbonic acid, which then dissociates into bicarbonate ions and hydrogen ions. This reaction is catalyzed by the enzyme carbonic anhydrase.
CO2 + H2O → H2CO3 → H+ + HCO3-
The bicarbonate ions are then transported to the lungs, where they are converted back into CO2 and exhaled.
Benefits of Bicarbonate Transport
The bicarbonate transport system has several benefits:
- Efficient: Bicarbonate transport is an efficient way to remove CO2 from tissues and transport it to the lungs.
- ** Buffering capacity**: Bicarbonate helps to buffer the pH of the blood, preventing acidosis.
- Regulation of CO2 levels: The bicarbonate transport system helps to regulate CO2 levels in the blood, which is essential for maintaining proper respiratory function.
Regulation of Bicarbonate Transport

The bicarbonate transport system is regulated by several factors, including:
- pH levels: Changes in pH levels can affect the activity of carbonic anhydrase and the transport of bicarbonate.
- CO2 levels: Changes in CO2 levels can affect the transport of bicarbonate.
- Hormonal regulation: Hormones such as adrenaline and insulin can affect the transport of bicarbonate.
Diseases Related to Bicarbonate Transport
Dysregulation of the bicarbonate transport system can lead to several diseases, including:
- Respiratory acidosis: A condition in which the blood becomes too acidic due to impaired CO2 removal.
- Metabolic acidosis: A condition in which the blood becomes too acidic due to impaired bicarbonate transport.
- Kidney disease: Impaired bicarbonate transport can lead to kidney disease.
Conclusion

In conclusion, the transportation of CO2 in the blood is a complex process that involves multiple steps and various forms of the gas. Bicarbonate is the primary form of CO2 transport in the blood, and its regulation is essential for maintaining proper respiratory function. Understanding the bicarbonate transport system can provide valuable insights into the development of diseases related to CO2 transport.
We hope this article has provided you with a comprehensive understanding of the bicarbonate transport system. If you have any questions or comments, please feel free to share them below.
What is the primary form of CO2 transport in the blood?
+Bicarbonate is the primary form of CO2 transport in the blood.
What enzyme catalyzes the reaction between CO2 and water to form carbonic acid?
+Carbonic anhydrase catalyzes the reaction between CO2 and water to form carbonic acid.
What is the benefit of bicarbonate transport in the blood?
+The bicarbonate transport system helps to regulate CO2 levels in the blood, preventing acidosis and maintaining proper respiratory function.