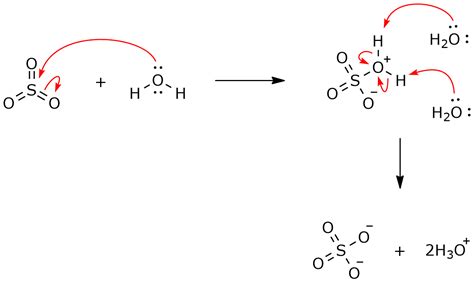
Sulfur trioxide, also known as sulfuric oxide or sulfur oxide, is a chemical compound with the formula SO3. It is a colorless, oily liquid that is highly reactive, particularly with water. When sulfur trioxide reacts with water, it forms sulfuric acid, which is a strong acid that is widely used in various industrial applications. In this article, we will explore three ways sulfur trioxide reacts with water and the resulting products.
What is Sulfur Trioxide?

Sulfur trioxide is a highly reactive gas that is produced by the combustion of sulfur or sulfur-containing compounds. It is a major air pollutant that can cause respiratory problems and other health issues. However, it is also an important industrial chemical that is used in the production of sulfuric acid, fertilizers, and other chemicals.
The Reaction of Sulfur Trioxide with Water

When sulfur trioxide reacts with water, it forms sulfuric acid, which is a strong acid that is widely used in various industrial applications. The reaction is highly exothermic, releasing a significant amount of heat energy. The resulting sulfuric acid is a clear, colorless liquid that is highly corrosive and can cause severe burns.
1. Sulfur Trioxide Reacts with Water to Form Sulfuric Acid
The most common way sulfur trioxide reacts with water is to form sulfuric acid. The reaction is as follows:
SO3 + H2O → H2SO4
This reaction is highly exothermic, releasing a significant amount of heat energy. The resulting sulfuric acid is a clear, colorless liquid that is highly corrosive and can cause severe burns.
Properties of Sulfuric Acid

Sulfuric acid is a strong acid that is widely used in various industrial applications. It is a clear, colorless liquid that is highly corrosive and can cause severe burns. It has a number of important properties, including:
- High acidity: Sulfuric acid is a strong acid that is highly corrosive and can cause severe burns.
- High boiling point: Sulfuric acid has a high boiling point, which makes it useful for a variety of industrial applications.
- High density: Sulfuric acid is a dense liquid that is heavier than water.
2. Sulfur Trioxide Reacts with Water to Form Sulfurous Acid
In addition to forming sulfuric acid, sulfur trioxide can also react with water to form sulfurous acid. The reaction is as follows:
2SO3 + H2O → H2S2O7
This reaction is less common than the reaction to form sulfuric acid, but it is still an important industrial process.
Properties of Sulfurous Acid

Sulfurous acid is a weak acid that is less corrosive than sulfuric acid. It has a number of important properties, including:
- Low acidity: Sulfurous acid is a weak acid that is less corrosive than sulfuric acid.
- Low boiling point: Sulfurous acid has a low boiling point, which makes it useful for a variety of industrial applications.
- Low density: Sulfurous acid is a less dense liquid than sulfuric acid.
3. Sulfur Trioxide Reacts with Water to Form Pyrosulfuric Acid
In addition to forming sulfuric acid and sulfurous acid, sulfur trioxide can also react with water to form pyrosulfuric acid. The reaction is as follows:
SO3 + H2SO4 → H2S2O7
This reaction is less common than the reactions to form sulfuric acid and sulfurous acid, but it is still an important industrial process.
Properties of Pyrosulfuric Acid

Pyrosulfuric acid is a strong acid that is similar to sulfuric acid. It has a number of important properties, including:
- High acidity: Pyrosulfuric acid is a strong acid that is highly corrosive and can cause severe burns.
- High boiling point: Pyrosulfuric acid has a high boiling point, which makes it useful for a variety of industrial applications.
- High density: Pyrosulfuric acid is a dense liquid that is heavier than water.
In conclusion, sulfur trioxide reacts with water in three different ways to form sulfuric acid, sulfurous acid, and pyrosulfuric acid. Each of these acids has its own unique properties and uses, and they are all important industrial chemicals.
If you have any questions or comments about the reaction of sulfur trioxide with water, please let us know in the comments section below.
What is sulfur trioxide?
+Sulfur trioxide is a chemical compound with the formula SO3. It is a colorless, oily liquid that is highly reactive, particularly with water.
What is the reaction of sulfur trioxide with water?
+The reaction of sulfur trioxide with water is highly exothermic, releasing a significant amount of heat energy. The resulting sulfuric acid is a clear, colorless liquid that is highly corrosive and can cause severe burns.
What are the properties of sulfuric acid?
+Sulfuric acid is a strong acid that is highly corrosive and can cause severe burns. It has a high boiling point and a high density, making it useful for a variety of industrial applications.