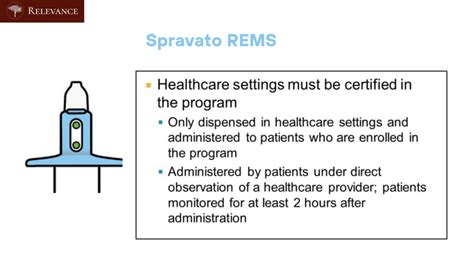
Spravato, a nasal spray medication containing esketamine, has been approved by the FDA for treatment-resistant depression (TRD) and depressive symptoms in adults with major depressive disorder (MDD) with suicidal thoughts or behavior. As part of the Risk Evaluation and Mitigation Strategy (REMS) program, healthcare providers and patients must adhere to specific guidelines to ensure safe use. Here are 5 essential Spravato REMS patient monitoring tips to ensure safe and effective treatment.
Understanding the Spravato REMS Program

The Spravato REMS program is designed to mitigate the risks associated with Spravato, including the potential for abuse, misuse, and serious adverse outcomes. As a healthcare provider, it is crucial to understand the program's requirements and implement them in your clinical practice.
Key Components of the Spravato REMS Program
- Patient enrollment and education
- Healthcare provider training and certification
- Pharmacy certification and dispensing
- Patient monitoring and follow-up
1. Patient Enrollment and Education

Before initiating Spravato treatment, patients must be enrolled in the REMS program and educated on the safe use of the medication. This includes:
- Reviewing the patient's medical history and current medications
- Discussing the potential risks and benefits of Spravato
- Providing instructions on proper administration and dosing
- Emphasizing the importance of follow-up appointments and monitoring
Key Patient Education Points
- Spravato is only approved for use in certified medical offices or clinics
- Patients must be accompanied by a caregiver or driver after treatment
- Spravato can cause dissociation, sedation, and increased blood pressure
- Patients should not drive or operate heavy machinery until the next day
2. Monitoring for Dissociation and Sedation

Dissociation and sedation are common adverse reactions associated with Spravato. Patients should be monitored closely for these symptoms during and after treatment.
- Assess patients for dissociation using standardized tools, such as the Clinician-Administered Dissociative States Scale (CADSS)
- Monitor patients for sedation and respiratory depression
- Ensure patients are accompanied by a caregiver or driver after treatment
Managing Dissociation and Sedation
- Provide a quiet and comfortable environment for patients during treatment
- Offer emotional support and reassurance to patients experiencing dissociation
- Consider administering sedation reversal agents, such as flumazenil, if necessary
3. Blood Pressure Monitoring

Spravato can cause significant increases in blood pressure, which can lead to serious cardiovascular events. Patients should be monitored closely for changes in blood pressure during and after treatment.
- Measure patients' blood pressure before, during, and after treatment
- Monitor patients for signs of cardiovascular instability, such as chest pain or shortness of breath
- Consider adjusting treatment or providing additional interventions, such as antihypertensive medications, if necessary
Managing Blood Pressure Increases
- Provide patients with instructions on proper blood pressure monitoring at home
- Consider referring patients to a cardiologist or primary care physician for ongoing blood pressure management
4. Suicidal Thoughts and Behaviors

Spravato is approved for use in patients with depressive symptoms and suicidal thoughts or behavior. Patients should be closely monitored for changes in mood and behavior during treatment.
- Assess patients for suicidal thoughts and behaviors using standardized tools, such as the Columbia-Suicide Severity Rating Scale (C-SSRS)
- Monitor patients for changes in mood and behavior during and after treatment
- Provide patients with crisis intervention resources, such as the National Suicide Prevention Lifeline (1-800-273-TALK)
Managing Suicidal Thoughts and Behaviors
- Provide patients with ongoing emotional support and reassurance
- Consider adjusting treatment or providing additional interventions, such as counseling or psychotherapy, if necessary
5. Long-Term Monitoring and Follow-Up

Long-term monitoring and follow-up are crucial for ensuring safe and effective treatment with Spravato. Patients should be seen regularly by their healthcare provider to assess treatment response and manage potential adverse reactions.
- Schedule regular follow-up appointments with patients to assess treatment response and manage potential adverse reactions
- Monitor patients for changes in mood and behavior during and after treatment
- Provide patients with ongoing emotional support and reassurance
Key Long-Term Monitoring Points
- Treatment response and efficacy
- Potential adverse reactions, such as dissociation, sedation, and increased blood pressure
- Changes in mood and behavior
By following these 5 essential Spravato REMS patient monitoring tips, healthcare providers can ensure safe and effective treatment for patients with treatment-resistant depression and depressive symptoms.