Phosphorus, a highly reactive nonmetal, is an essential element in various biological processes, including DNA and ATP production. Understanding the electron configuration of phosphorus is crucial in chemistry and physics, as it helps explain the element's unique properties and behavior. In this article, we will break down the phosphorus electron configuration into 5 easy steps, making it simple to grasp, even for those new to chemistry.
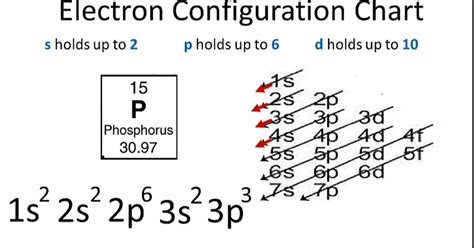
Phosphorus is the 15th element on the periodic table, with an atomic number of 15 and an atomic mass of 30.97 u. It is a member of the nitrogen group and is highly reactive due to its unique electron configuration. The electron configuration of an atom is the arrangement of electrons in its orbitals, which determines the atom's chemical properties and behavior.

Step 1: Understanding the Aufbau Principle
The Aufbau principle states that electrons occupy the lowest available energy levels in an atom. This principle is essential in understanding the electron configuration of phosphorus. The Aufbau principle is based on the idea that electrons fill the lowest available orbitals, which are the s, p, d, and f orbitals.
Order of Orbital Filling
The order of orbital filling is as follows:
- 1s orbital ( lowest energy level)
- 2s orbital
- 2p orbital
- 3s orbital
- 3p orbital
- 4s orbital
- 3d orbital

Step 2: Understanding the Pauli Exclusion Principle
The Pauli exclusion principle states that each orbital can hold a maximum of two electrons, which must have opposite spins. This principle is crucial in understanding the electron configuration of phosphorus, as it determines the number of electrons that can occupy each orbital.
Electron Spin
Electron spin is a fundamental property of electrons, which determines their behavior in an orbital. Electrons can have either an "up" or "down" spin, and when two electrons occupy the same orbital, they must have opposite spins.

Step 3: Determining the Electron Configuration of Phosphorus
To determine the electron configuration of phosphorus, we need to apply the Aufbau principle and the Pauli exclusion principle. Phosphorus has an atomic number of 15, which means it has 15 electrons. The electron configuration of phosphorus is as follows:
1s² 2s² 2p⁶ 3s² 3p³
The electron configuration shows that the 1s, 2s, and 2p orbitals are fully occupied, while the 3s and 3p orbitals are partially occupied.

Step 4: Understanding the Valence Electrons of Phosphorus
The valence electrons of an atom are the electrons in the outermost energy level, which participate in chemical bonding. Phosphorus has five valence electrons, which are the electrons in the 3s and 3p orbitals.
Valence Electron Configuration
The valence electron configuration of phosphorus is as follows:
3s² 3p³
The valence electron configuration shows that phosphorus has three unpaired electrons in the 3p orbital, which makes it highly reactive.

Step 5: Understanding the Chemical Properties of Phosphorus
The electron configuration of phosphorus determines its chemical properties and behavior. Phosphorus is highly reactive due to its unique electron configuration, which makes it an essential element in various biological processes.
Chemical Properties
Some of the chemical properties of phosphorus include:
- Highly reactive
- Forms covalent bonds
- Participates in biological processes
- Forms phosphates and phosphoric acid

In conclusion, understanding the electron configuration of phosphorus is essential in chemistry and physics. By applying the Aufbau principle and the Pauli exclusion principle, we can determine the electron configuration of phosphorus, which is 1s² 2s² 2p⁶ 3s² 3p³. The valence electrons of phosphorus are highly reactive, making it an essential element in various biological processes.
Now that you have learned about the phosphorus electron configuration, we encourage you to share your thoughts and questions in the comments section below. What other topics in chemistry would you like to learn about?
What is the atomic number of phosphorus?
+The atomic number of phosphorus is 15.
What is the electron configuration of phosphorus?
+The electron configuration of phosphorus is 1s² 2s² 2p⁶ 3s² 3p³.
Why is phosphorus highly reactive?
+Phosphorus is highly reactive due to its unique electron configuration, which makes it have three unpaired electrons in the 3p orbital.