The fascinating world of chemistry! Understanding the electron configuration of elements is a fundamental concept in chemistry, and today we're going to explore the manganese electron configuration. Manganese, with its atomic number 25, is a transition metal that plays a crucial role in various biological and industrial processes. In this article, we'll break down the manganese electron configuration into 3 simple steps, making it easy to grasp for students and enthusiasts alike.
Step 1: Understanding the Atomic Number and Electron Shells

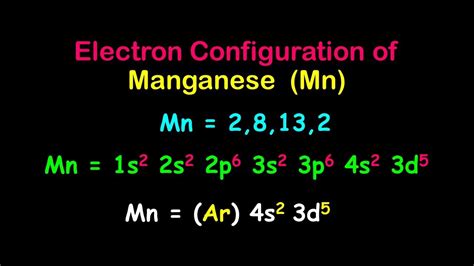
The atomic number of manganese is 25, which means it has 25 protons in its atomic nucleus. To determine the electron configuration, we need to consider the number of electrons in each energy level or electron shell. The electron shells are filled in a specific order, following the Aufbau principle and the Pauli Exclusion Principle. The first two electron shells (1s and 2s) can hold a maximum of 2 electrons each, while the third shell (3s, 3p, and 3d) can hold up to 18 electrons.
Electron Shell Configuration
- 1s: 2 electrons
- 2s: 2 electrons
- 2p: 6 electrons
- 3s: 2 electrons
- 3p: 6 electrons
- 3d: 5 electrons (in the case of manganese)
Step 2: Filling the Electron Shells

Now that we know the electron shell configuration, let's fill the shells with electrons. The electrons occupy the lowest available energy levels, and each orbital can hold a maximum of two electrons with opposite spins. The 1s, 2s, and 2p orbitals are filled first, followed by the 3s and 3p orbitals. The 3d orbital, which is the outermost energy level, is filled last.
Electron Configuration of Manganese
- 1s: 2 electrons (1s²)
- 2s: 2 electrons (2s²)
- 2p: 6 electrons (2p⁶)
- 3s: 2 electrons (3s²)
- 3p: 6 electrons (3p⁶)
- 3d: 5 electrons (3d⁵)
- 4s: 2 electrons (4s²)
The electron configuration of manganese is [Ar] 3d⁵ 4s².
Step 3: Understanding the Significance of Electron Configuration

The electron configuration of manganese plays a crucial role in its chemical properties and reactivity. The 3d orbital, which is half-filled, makes manganese a highly reactive element. The 4s electrons, which are in the outermost energy level, participate in chemical bonding and reactions.
Applications of Manganese Electron Configuration
- Manganese is used in steel production, as it helps to remove impurities and improve the strength of steel.
- Manganese is a key component in the production of dry cell batteries, such as alkaline batteries.
- Manganese is used in the manufacture of glass, ceramics, and other materials.
In conclusion, understanding the manganese electron configuration is essential for grasping its chemical properties and reactivity. By breaking down the electron configuration into 3 simple steps, we can appreciate the complexity and beauty of the atomic structure of manganese.
What is the atomic number of manganese?
+The atomic number of manganese is 25.
What is the electron configuration of manganese?
+The electron configuration of manganese is [Ar] 3d⁵ 4s².
What is the significance of the 3d orbital in manganese?
+The 3d orbital, which is half-filled, makes manganese a highly reactive element.