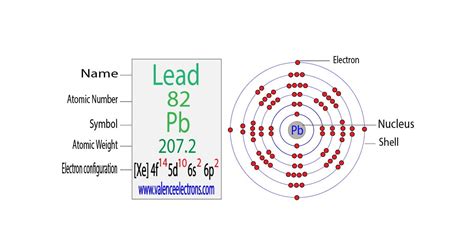
The concept of electron configuration is crucial in understanding the behavior of atoms and molecules. One of the most widely used and important electron configurations is the lead electron configuration. Lead, a chemical element with the symbol Pb and atomic number 82, is a post-transition metal that is widely used in various applications, including batteries, radiation shielding, and pigments. In this article, we will delve into the world of lead electron configuration, exploring its importance, benefits, and applications.
Understanding Electron Configuration
Before diving into the specifics of lead electron configuration, it's essential to understand the basics of electron configuration. Electron configuration refers to the arrangement of electrons in an atom, which is determined by the number of protons in the atomic nucleus. The electrons are arranged in energy levels or shells, with each shell having a specific capacity. The electrons in an atom are arranged in a specific order, with the electrons in the outermost energy level being the most reactive.

Lead Electron Configuration: A Deep Dive
Lead, with an atomic number of 82, has a unique electron configuration that sets it apart from other elements. The electron configuration of lead is:
1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p⁶ 4d¹⁰ 4f¹⁴ 5s² 5p⁶ 5d¹⁰ 6s² 6p²
This electron configuration shows that lead has a full outer energy level, with the electrons in the outermost energy level being arranged in a specific order. The electrons in the outermost energy level are the most reactive, and they play a crucial role in determining the chemical properties of lead.

Importance of Lead Electron Configuration
The lead electron configuration is essential in understanding the chemical and physical properties of lead. The electron configuration of lead determines its reactivity, which is crucial in various applications, including:
- Battery Production: Lead is widely used in the production of batteries, particularly in the manufacture of lead-acid batteries. The electron configuration of lead plays a crucial role in determining its reactivity, which is essential for the functioning of these batteries.
- Radiation Shielding: Lead is used as a radiation shield due to its high density and high atomic number. The electron configuration of lead determines its ability to absorb radiation, making it an effective radiation shield.
- Pigments: Lead is used as a pigment in various applications, including paint and ceramics. The electron configuration of lead determines its color and opacity, making it an essential component in these applications.
Benefits of Understanding Lead Electron Configuration
Understanding the lead electron configuration has numerous benefits, including:
- Improved Materials Science: Understanding the electron configuration of lead can help improve materials science, particularly in the development of new materials and technologies.
- Enhanced Battery Performance: Understanding the electron configuration of lead can help improve battery performance, particularly in the development of more efficient and sustainable batteries.
- Radiation Protection: Understanding the electron configuration of lead can help improve radiation protection, particularly in the development of more effective radiation shields.
Applications of Lead Electron Configuration
The lead electron configuration has various applications, including:
- Battery Production: Lead is widely used in the production of batteries, particularly in the manufacture of lead-acid batteries.
- Radiation Shielding: Lead is used as a radiation shield due to its high density and high atomic number.
- Pigments: Lead is used as a pigment in various applications, including paint and ceramics.

Conclusion: The Significance of Lead Electron Configuration
In conclusion, the lead electron configuration is a crucial concept in understanding the behavior of lead atoms and molecules. The electron configuration of lead determines its reactivity, which is essential in various applications, including battery production, radiation shielding, and pigments. Understanding the lead electron configuration has numerous benefits, including improved materials science, enhanced battery performance, and radiation protection. The applications of lead electron configuration are diverse, ranging from battery production to radiation shielding and pigments.
Call to Action: Share Your Thoughts
We hope this article has provided you with a comprehensive understanding of the lead electron configuration. Share your thoughts and comments below, and let's continue the conversation. Do you have any questions or topics you'd like to discuss? Share them with us, and we'll do our best to provide you with more information.
What is the electron configuration of lead?
+The electron configuration of lead is 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p⁶ 4d¹⁰ 4f¹⁴ 5s² 5p⁶ 5d¹⁰ 6s² 6p².
What are the applications of lead electron configuration?
+The applications of lead electron configuration include battery production, radiation shielding, and pigments.
Why is understanding lead electron configuration important?
+Understanding lead electron configuration is important because it determines the reactivity of lead, which is essential in various applications.