Iron is a highly reactive metal that readily combines with oxygen to form a variety of oxides. One of the most common and stable of these oxides is iron(III) oxide, also known as ferric oxide. This compound is widely encountered in nature and has numerous applications in various industries.
When iron reacts with oxygen, it undergoes a process known as oxidation, which involves the loss of electrons to form a positive ion, also known as a cation. In the case of iron, the reaction with oxygen results in the formation of iron(III) ions, which then combine with oxygen ions to form iron(III) oxide. This reaction can occur through a variety of mechanisms, including thermal decomposition, electrolysis, and chemical synthesis.

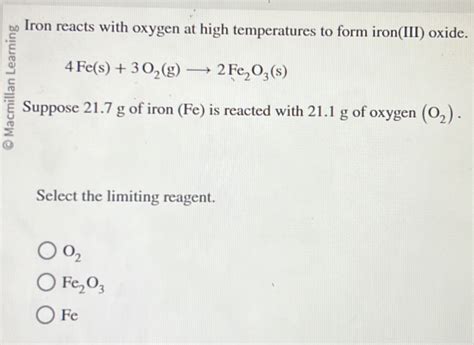
The reaction between iron and oxygen to form iron(III) oxide can be represented by the following equation:
4Fe (s) + 3O2 (g) → 2Fe2O3 (s)
In this equation, four atoms of iron react with three molecules of oxygen to form two molecules of iron(III) oxide. This reaction is highly exothermic, releasing a significant amount of heat energy.
Properties of Iron(III) Oxide
Iron(III) oxide is a reddish-brown powder with a number of distinct properties. It is highly insoluble in water and has a melting point of around 1565°C. It is also highly stable and resistant to corrosion, making it a useful material in a variety of applications.
Some of the key properties of iron(III) oxide include:
- Chemical formula: Fe2O3
- Molecular weight: 159.69 g/mol
- Appearance: Reddish-brown powder
- Melting point: 1565°C
- Boiling point: 2800°C
- Solubility: Insoluble in water

Preparation of Iron(III) Oxide
Iron(III) oxide can be prepared through a variety of methods, including thermal decomposition, electrolysis, and chemical synthesis. One common method involves the reaction of iron with oxygen in the presence of heat.
Here are the steps involved in preparing iron(III) oxide through thermal decomposition:
- Obtain a sample of iron filings or powder.
- Heat the iron in a crucible or furnace until it reaches a temperature of around 500°C.
- Allow the iron to react with oxygen in the air for several hours.
- Remove the crucible from the heat source and allow it to cool.
- Collect the resulting iron(III) oxide powder.

Applications of Iron(III) Oxide
Iron(III) oxide has a wide range of applications in various industries, including:
- Pigments: Iron(III) oxide is used as a pigment in paints, coatings, and plastics.
- Catalysts: Iron(III) oxide is used as a catalyst in the production of ammonia and other chemicals.
- Pharmaceuticals: Iron(III) oxide is used as an excipient in some pharmaceutical applications.
- Cosmetics: Iron(III) oxide is used as a coloring agent in some cosmetics.

Environmental Impact of Iron(III) Oxide
Iron(III) oxide is generally considered to be non-toxic and environmentally friendly. However, it can have some negative impacts on the environment if not disposed of properly.
Some of the potential environmental impacts of iron(III) oxide include:
- Soil contamination: Iron(III) oxide can contaminate soil and groundwater if not disposed of properly.
- Air pollution: Iron(III) oxide can contribute to air pollution if it is released into the atmosphere during industrial processes.
- Water pollution: Iron(III) oxide can contaminate waterways if it is released into the environment.

Conclusion
In conclusion, iron(III) oxide is a widely encountered compound with a range of properties and applications. It is prepared through the reaction of iron with oxygen and has a number of distinct properties, including its chemical formula, molecular weight, and melting point. Iron(III) oxide has a wide range of applications in various industries, including pigments, catalysts, and pharmaceuticals. However, it can also have some negative impacts on the environment if not disposed of properly.
We hope you have enjoyed this article and have learned something new about iron(III) oxide. If you have any questions or comments, please feel free to leave them in the section below.
What is iron(III) oxide?
+Iron(III) oxide is a compound with the chemical formula Fe2O3. It is a reddish-brown powder that is highly insoluble in water and has a melting point of around 1565°C.
How is iron(III) oxide prepared?
+Iron(III) oxide can be prepared through a variety of methods, including thermal decomposition, electrolysis, and chemical synthesis. One common method involves the reaction of iron with oxygen in the presence of heat.
What are the applications of iron(III) oxide?
+Iron(III) oxide has a wide range of applications in various industries, including pigments, catalysts, pharmaceuticals, and cosmetics.