Iron and oxygen are two of the most abundant elements on Earth, and when they react, they form a compound that is essential to many industrial and biological processes. Iron III oxide, also known as ferric oxide or rust, is the product of the reaction between iron and oxygen. In this article, we will delve into the details of this reaction, its mechanism, and its significance in various fields.

Iron is a highly reactive metal that readily loses its electrons to form ions. When iron is exposed to oxygen, it undergoes a redox reaction, where the iron atom loses its electrons to the oxygen molecule. This reaction is highly exothermic, releasing a significant amount of energy in the form of heat.
The Reaction Mechanism
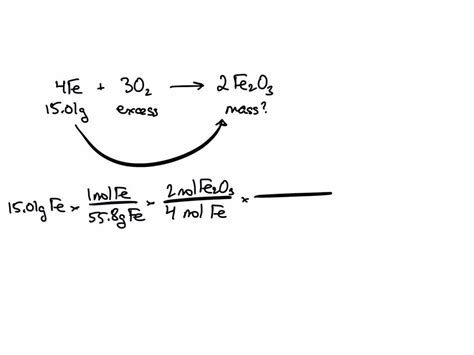
The reaction between iron and oxygen is a complex process that involves multiple steps. The overall reaction is represented by the following equation:
4Fe (s) + 3O2 (g) → 2Fe2O3 (s)
The reaction mechanism involves the formation of iron(II) ions, which then react with oxygen to form iron(III) ions. These ions then combine to form iron III oxide.

Step 1: Formation of Iron(II) Ions
The first step in the reaction is the formation of iron(II) ions. This occurs when iron atoms lose two electrons to form a stable ion.
Fe (s) → Fe2+ (aq) + 2e-
Step 2: Reaction with Oxygen
The iron(II) ions then react with oxygen molecules to form iron(III) ions.
Fe2+ (aq) + O2 (g) → Fe3+ (aq) + O2- (aq)
Step 3: Formation of Iron III Oxide
The iron(III) ions then combine with oxygen ions to form iron III oxide.
2Fe3+ (aq) + 3O2- (aq) → Fe2O3 (s)
Significance of the Reaction
The reaction between iron and oxygen is essential to many industrial and biological processes. Iron III oxide is a crucial component in the production of steel, which is used in construction, transportation, and many other industries. It is also used as a pigment in paints, coatings, and ceramics.

In addition, the reaction between iron and oxygen is also important in biological systems. Iron is an essential nutrient for many living organisms, and the reaction with oxygen helps to regulate its availability.
Biological Significance
Iron is a crucial element for many biological processes, including oxygen transport, DNA synthesis, and enzyme function. The reaction between iron and oxygen helps to regulate the availability of iron in the body.

In conclusion, the reaction between iron and oxygen is a complex process that is essential to many industrial and biological processes. Understanding the mechanism of this reaction is crucial for developing new technologies and improving our knowledge of biological systems.
We encourage you to share your thoughts and questions about the reaction between iron and oxygen in the comments section below. How do you think this reaction impacts our daily lives?
What is the product of the reaction between iron and oxygen?
+The product of the reaction between iron and oxygen is iron III oxide, also known as ferric oxide or rust.
What is the significance of the reaction between iron and oxygen in biological systems?
+The reaction between iron and oxygen helps to regulate the availability of iron in the body, which is essential for many biological processes, including oxygen transport, DNA synthesis, and enzyme function.
What is the industrial significance of the reaction between iron and oxygen?
+The reaction between iron and oxygen is essential for the production of steel, which is used in construction, transportation, and many other industries. Iron III oxide is also used as a pigment in paints, coatings, and ceramics.