The world of chemistry can be complex and overwhelming, but understanding the basics of forming ions is essential for any student or enthusiast. Ions are atoms or molecules that have gained or lost electrons, resulting in a net positive or negative charge. In this article, we'll explore the process of electron addition and removal, making it easy for you to grasp this fundamental concept in chemistry.
Atoms are the building blocks of matter, and they consist of protons, neutrons, and electrons. Protons and neutrons reside in the nucleus, while electrons orbit around it. The number of protons in an atom determines its atomic number, which defines the element. However, the number of electrons can vary, leading to the formation of ions. In this article, we'll delve into the process of forming ions through electron addition and removal.
Understanding Electron Configuration

To comprehend ion formation, it's essential to understand electron configuration. Electron configuration refers to the arrangement of electrons in an atom. The electrons occupy specific energy levels or shells, which are further divided into subshells. The number of electrons in an atom is equal to the number of protons, and this number determines the element's position in the periodic table.
Valence Electrons and Ion Formation
Valence electrons are the electrons in the outermost energy level of an atom. These electrons participate in chemical bonding and reactions. When an atom gains or loses electrons, it forms an ion. The number of valence electrons an atom has determines its tendency to form ions. Atoms with a full outer energy level tend to be stable and less reactive, while those with partially filled outer energy levels are more reactive.
Electron Addition: Forming Negative Ions

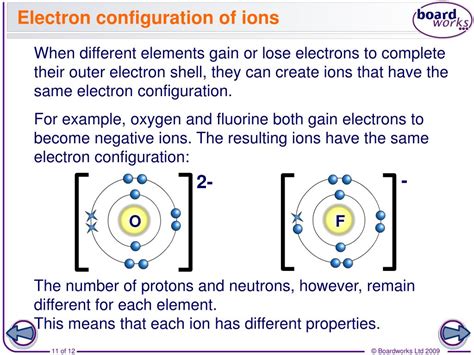
When an atom gains one or more electrons, it forms a negative ion, also known as an anion. This process is called electron addition. The added electrons occupy the outermost energy level, resulting in a net negative charge. The number of electrons gained by an atom determines the charge of the anion. For example, when a chlorine atom gains one electron, it forms a chloride ion with a -1 charge.
Examples of Electron Addition
- Chlorine (Cl) + 1 electron → Chloride ion (Cl-) with a -1 charge
- Oxygen (O) + 2 electrons → Oxide ion (O2-) with a -2 charge
- Nitrogen (N) + 3 electrons → Nitride ion (N3-) with a -3 charge
Electron Removal: Forming Positive Ions

When an atom loses one or more electrons, it forms a positive ion, also known as a cation. This process is called electron removal. The lost electrons come from the outermost energy level, resulting in a net positive charge. The number of electrons lost by an atom determines the charge of the cation. For example, when a sodium atom loses one electron, it forms a sodium ion with a +1 charge.
Examples of Electron Removal
- Sodium (Na) - 1 electron → Sodium ion (Na+) with a +1 charge
- Magnesium (Mg) - 2 electrons → Magnesium ion (Mg2+) with a +2 charge
- Aluminum (Al) - 3 electrons → Aluminum ion (Al3+) with a +3 charge
Ion Formation in Compounds

Ion formation is not limited to individual atoms; it also occurs in compounds. When two or more atoms share electrons to form a chemical bond, they can form ions. This process is called ionic bonding. In ionic compounds, one atom loses electrons to form a cation, while another atom gains electrons to form an anion. The resulting ions are electrostatically attracted to each other, forming a strong chemical bond.
Examples of Ionic Compounds
- Sodium chloride (NaCl): Sodium (Na+) and chloride (Cl-) ions
- Calcium carbonate (CaCO3): Calcium (Ca2+) and carbonate (CO32-) ions
- Potassium nitrate (KNO3): Potassium (K+) and nitrate (NO3-) ions
Conclusion
Forming ions is a fundamental concept in chemistry that involves the addition or removal of electrons from atoms. Understanding electron configuration and the process of electron addition and removal is essential for grasping ion formation. Ions play a crucial role in chemical bonding and reactions, and their formation is a critical aspect of chemistry.Now that you've read this article, take a moment to reflect on what you've learned. Do you have any questions or topics you'd like to explore further? Share your thoughts and comments below!
What is the difference between a cation and an anion?
+A cation is a positively charged ion formed when an atom loses one or more electrons. An anion is a negatively charged ion formed when an atom gains one or more electrons.
What is the process of electron addition?
+Electron addition is the process by which an atom gains one or more electrons, resulting in the formation of a negative ion or anion.
What is ionic bonding?
+Ionic bonding is the process by which two or more atoms share electrons to form a chemical bond, resulting in the formation of ions. One atom loses electrons to form a cation, while another atom gains electrons to form an anion.