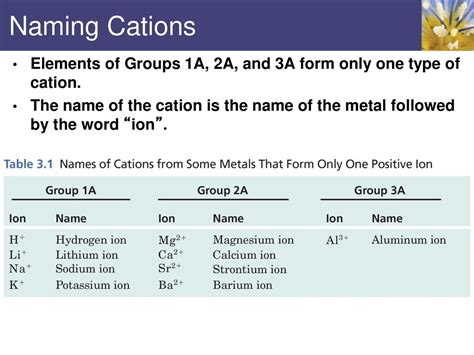
Cations are positively charged ions that are formed when an atom loses one or more electrons. While most cations are formed from single elements, there are some exceptions where multiple elements combine to form a single cation. In this article, we will explore three elements that form only one cation, discussing their properties, characteristics, and examples.

What are Cations?
Before diving into the three elements that form only one cation, it's essential to understand what cations are and how they are formed. Cations are positively charged ions that are created when an atom loses one or more electrons. This process is known as ionization. Cations can be formed from single elements, such as sodium (Na+) or calcium (Ca2+), or from compounds, such as ammonium (NH4+) or hydronium (H3O+).
Characteristics of Cations
Cations have several characteristics that distinguish them from anions (negatively charged ions). Some of the key characteristics of cations include:
- Positive charge: Cations have a positive charge due to the loss of one or more electrons.
- Small size: Cations are typically smaller than the original atom due to the loss of electrons.
- High reactivity: Cations are highly reactive due to their positive charge, which makes them attract electrons from other atoms or molecules.
Element 1: Ammonium (NH4+)

The ammonium ion (NH4+) is a cation that is formed from the combination of nitrogen and hydrogen atoms. Ammonium is a polyatomic ion, meaning it is composed of multiple atoms. It has a positive charge due to the loss of one electron from the nitrogen atom.
Ammonium is commonly found in fertilizers, cleaning products, and pharmaceuticals. It is also a key component of amino acids, which are the building blocks of proteins.
Properties of Ammonium
Some of the key properties of ammonium include:
- Positive charge: Ammonium has a positive charge due to the loss of one electron from the nitrogen atom.
- High reactivity: Ammonium is highly reactive due to its positive charge, which makes it attract electrons from other atoms or molecules.
- Solubility: Ammonium is highly soluble in water, which makes it useful in various applications.
Element 2: Hydronium (H3O+)

The hydronium ion (H3O+) is a cation that is formed from the combination of hydrogen and oxygen atoms. Hydronium is a polyatomic ion, meaning it is composed of multiple atoms. It has a positive charge due to the loss of one electron from the oxygen atom.
Hydronium is commonly found in acidic solutions, such as hydrochloric acid (HCl) or sulfuric acid (H2SO4). It is also a key component of the proton exchange membrane (PEM) fuel cell.
Properties of Hydronium
Some of the key properties of hydronium include:
- Positive charge: Hydronium has a positive charge due to the loss of one electron from the oxygen atom.
- High reactivity: Hydronium is highly reactive due to its positive charge, which makes it attract electrons from other atoms or molecules.
- Acidic properties: Hydronium is a key component of acidic solutions, which makes it useful in various applications.
Element 3: Hydroxylammonium (NH3OH+)

The hydroxylammonium ion (NH3OH+) is a cation that is formed from the combination of nitrogen, oxygen, and hydrogen atoms. Hydroxylammonium is a polyatomic ion, meaning it is composed of multiple atoms. It has a positive charge due to the loss of one electron from the nitrogen atom.
Hydroxylammonium is commonly found in cleaning products, such as disinfectants and sanitizers. It is also a key component of the production of polyurethane foams.
Properties of Hydroxylammonium
Some of the key properties of hydroxylammonium include:
- Positive charge: Hydroxylammonium has a positive charge due to the loss of one electron from the nitrogen atom.
- High reactivity: Hydroxylammonium is highly reactive due to its positive charge, which makes it attract electrons from other atoms or molecules.
- Solubility: Hydroxylammonium is highly soluble in water, which makes it useful in various applications.
What is a cation?
+A cation is a positively charged ion that is formed when an atom loses one or more electrons.
What are the characteristics of cations?
+Cations have a positive charge, are typically small in size, and are highly reactive due to their positive charge.
What are the three elements that form only one cation?
+The three elements that form only one cation are ammonium (NH4+), hydronium (H3O+), and hydroxylammonium (NH3OH+).
In conclusion, the three elements that form only one cation are ammonium, hydronium, and hydroxylammonium. These cations have unique properties and characteristics that make them useful in various applications. Understanding the properties and characteristics of cations is essential for understanding various chemical reactions and processes.
We hope you found this article informative and helpful. If you have any questions or comments, please feel free to share them with us. Don't forget to share this article with your friends and colleagues who may be interested in learning more about cations.