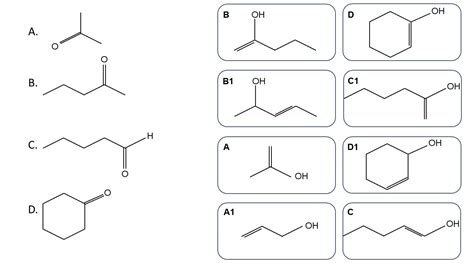
The keto-enol tautomerism is a fundamental concept in organic chemistry, and identifying the keto form of enol tautomers is a crucial skill for any chemistry enthusiast. In this article, we will delve into the world of keto-enol tautomerism and explore five ways to identify the keto form of enol tautomers.
Understanding Keto-Enol Tautomerism

Keto-enol tautomerism is a process where a molecule exists in two interconvertible forms: the keto form and the enol form. The keto form is a carbonyl compound, while the enol form is an alkene with a hydroxyl group attached to one of the carbon atoms. This interconversion is catalyzed by acid or base, and it plays a crucial role in many organic reactions.
Importance of Identifying Keto Form of Enol Tautomers
Identifying the keto form of enol tautomers is essential in organic chemistry because it helps predict the reactivity and stability of a molecule. The keto form is generally more stable than the enol form, and understanding the keto form can help chemists design and synthesize new compounds.
5 Ways to Identify Keto Form of Enol Tautomers
Now that we have a basic understanding of keto-enol tautomerism, let's dive into the five ways to identify the keto form of enol tautomers.
1. Look for the Carbonyl Group
The keto form of an enol tautomer is characterized by the presence of a carbonyl group (C=O). This group is usually easy to spot in a molecule, and its presence is a strong indication that the molecule is in its keto form.

2. Check for the Presence of an Alpha Hydrogen
The enol form of a tautomer has an alpha hydrogen atom, which is a hydrogen atom attached to the carbon atom adjacent to the carbonyl group. If the molecule does not have an alpha hydrogen atom, it is likely in its keto form.
Example
For example, consider the molecule 2-butanone. This molecule has an alpha hydrogen atom, which indicates that it can exist in both keto and enol forms. However, the keto form is more stable and is the preferred form of the molecule.

3. Use NMR Spectroscopy
Nuclear magnetic resonance (NMR) spectroscopy is a powerful tool for identifying the keto form of enol tautomers. The keto form typically shows a singlet peak in the NMR spectrum, while the enol form shows a multiplet peak.
Example
For example, consider the molecule acetone. The keto form of acetone shows a singlet peak at 2.1 ppm in the NMR spectrum, while the enol form shows a multiplet peak at 4.5 ppm.

4. Use IR Spectroscopy
Infrared (IR) spectroscopy is another useful tool for identifying the keto form of enol tautomers. The keto form typically shows a strong absorption peak at 1710-1720 cm^-1, while the enol form shows a weaker absorption peak at 1640-1650 cm^-1.
Example
For example, consider the molecule benzophenone. The keto form of benzophenone shows a strong absorption peak at 1715 cm^-1 in the IR spectrum, while the enol form shows a weaker absorption peak at 1645 cm^-1.

5. Use Computational Methods
Computational methods such as density functional theory (DFT) can be used to predict the keto form of enol tautomers. These methods can provide detailed information about the molecular structure and energy of the keto form.
Example
For example, consider the molecule malonaldehyde. Computational methods such as DFT can be used to predict the keto form of malonaldehyde, which is the more stable form of the molecule.

Conclusion
In conclusion, identifying the keto form of enol tautomers is a crucial skill in organic chemistry. By using the five methods outlined in this article, chemists can predict the reactivity and stability of a molecule and design and synthesize new compounds.
We hope this article has been informative and helpful. Do you have any questions or comments about keto-enol tautomerism? Please share your thoughts in the comments section below.
What is keto-enol tautomerism?
+Keto-enol tautomerism is a process where a molecule exists in two interconvertible forms: the keto form and the enol form.
Why is it important to identify the keto form of enol tautomers?
+Identifying the keto form of enol tautomers is essential in organic chemistry because it helps predict the reactivity and stability of a molecule.
What are some common methods for identifying the keto form of enol tautomers?
+Some common methods for identifying the keto form of enol tautomers include looking for the carbonyl group, checking for the presence of an alpha hydrogen, using NMR spectroscopy, using IR spectroscopy, and using computational methods.