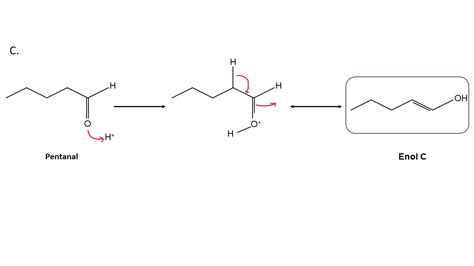
In organic chemistry, the enol form of keto tautomer is a crucial concept that plays a significant role in understanding various chemical reactions and mechanisms. The enol form is a tautomer of the keto form, and it is essential to be able to identify and distinguish between these two forms. In this article, we will explore five ways to identify the enol form of keto tautomer, along with examples and explanations.
Understanding Keto and Enol Tautomers

Keto and enol tautomers are two different forms of the same compound, which differ in the arrangement of their atoms. The keto form has a carbonyl group (C=O), while the enol form has a hydroxyl group (OH) attached to a carbon atom that is adjacent to a double bond. The enol form is typically more stable than the keto form due to the conjugation of the double bond with the hydroxyl group.
1. Structural Analysis
The first step in identifying the enol form of keto tautomer is to analyze the structure of the compound. Look for the presence of a hydroxyl group (OH) attached to a carbon atom that is adjacent to a double bond. This arrangement is characteristic of the enol form.
For example, consider the compound 2-buten-2-ol. The structure of this compound is:
CH₃CH=CHCH₂OH
In this compound, the hydroxyl group (OH) is attached to the carbon atom that is adjacent to the double bond, indicating that it is in the enol form.
Reactivity of Enol Form

The enol form of keto tautomer is more reactive than the keto form due to the presence of the hydroxyl group. The enol form can participate in various chemical reactions, such as nucleophilic addition reactions and electrophilic substitution reactions.
2. IR Spectroscopy
IR spectroscopy is a useful technique for identifying the enol form of keto tautomer. The enol form typically shows a broad absorption band in the range of 3200-3600 cm⁻¹ due to the presence of the hydroxyl group. This absorption band is characteristic of the enol form and can be used to distinguish it from the keto form.
For example, consider the IR spectrum of 2-buten-2-ol. The spectrum shows a broad absorption band at 3400 cm⁻¹, indicating the presence of the hydroxyl group and confirming that the compound is in the enol form.
NMR Spectroscopy

NMR spectroscopy is another useful technique for identifying the enol form of keto tautomer. The enol form typically shows a signal in the ¹H NMR spectrum due to the presence of the hydroxyl group. This signal is typically broad and appears in the range of 3-5 ppm.
For example, consider the ¹H NMR spectrum of 2-buten-2-ol. The spectrum shows a broad signal at 4.5 ppm due to the presence of the hydroxyl group, indicating that the compound is in the enol form.
3. Chemical Reactions
The enol form of keto tautomer can participate in various chemical reactions, such as nucleophilic addition reactions and electrophilic substitution reactions. These reactions can be used to identify the enol form and distinguish it from the keto form.
For example, consider the reaction of 2-buten-2-ol with sodium borohydride (NaBH₄). The reaction produces a secondary alcohol, indicating that the compound is in the enol form.
Stability of Enol Form

The enol form of keto tautomer is typically more stable than the keto form due to the conjugation of the double bond with the hydroxyl group. This stability can be used to identify the enol form and distinguish it from the keto form.
For example, consider the compound 2-buten-2-ol. The enol form of this compound is more stable than the keto form due to the conjugation of the double bond with the hydroxyl group.
4. Stereochemistry
The enol form of keto tautomer can exhibit stereochemistry, which can be used to identify the enol form and distinguish it from the keto form. The enol form typically shows a higher degree of stereochemistry than the keto form due to the presence of the hydroxyl group.
For example, consider the compound 2-buten-2-ol. The enol form of this compound shows a higher degree of stereochemistry than the keto form due to the presence of the hydroxyl group.
Biological Importance

The enol form of keto tautomer plays a crucial role in various biological processes, such as metabolism and biosynthesis. The enol form can participate in various enzyme-catalyzed reactions, which are essential for the proper functioning of living organisms.
For example, consider the compound pyruvate, which is a key intermediate in glycolysis. The enol form of pyruvate plays a crucial role in the conversion of pyruvate to acetyl-CoA, which is an essential step in the citric acid cycle.
5. Computational Methods
Computational methods, such as density functional theory (DFT), can be used to identify the enol form of keto tautomer. These methods can provide information on the structure, reactivity, and stability of the enol form, which can be used to distinguish it from the keto form.
For example, consider the compound 2-buten-2-ol. DFT calculations can provide information on the structure and reactivity of the enol form, which can be used to confirm that the compound is in the enol form.
We hope this article has provided you with a comprehensive understanding of the enol form of keto tautomer and how to identify it. The enol form plays a crucial role in various chemical reactions and biological processes, and its identification is essential for understanding these processes.
We encourage you to share your thoughts and questions in the comments section below. Do you have any experience with identifying the enol form of keto tautomer? Share your experiences and tips with us!
What is the main difference between the keto and enol forms of a compound?
+The main difference between the keto and enol forms of a compound is the arrangement of their atoms. The keto form has a carbonyl group (C=O), while the enol form has a hydroxyl group (OH) attached to a carbon atom that is adjacent to a double bond.
How can I identify the enol form of a compound using IR spectroscopy?
+The enol form of a compound typically shows a broad absorption band in the range of 3200-3600 cm⁻¹ due to the presence of the hydroxyl group. This absorption band is characteristic of the enol form and can be used to distinguish it from the keto form.
What is the biological importance of the enol form of keto tautomer?
+The enol form of keto tautomer plays a crucial role in various biological processes, such as metabolism and biosynthesis. The enol form can participate in various enzyme-catalyzed reactions, which are essential for the proper functioning of living organisms.