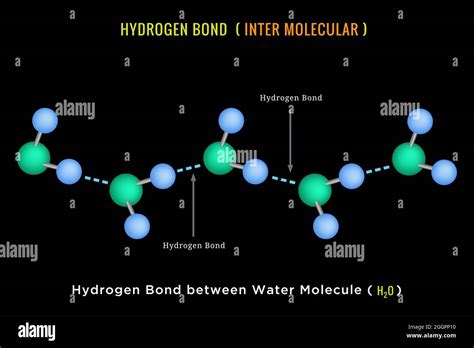
Hydrogen bonds play a crucial role in the structure and properties of water molecules. These weak electrostatic attractions between molecules are responsible for many of the unique characteristics of water, including its high surface tension, boiling point, and ability to dissolve a wide variety of substances. In this article, we will explore the importance of hydrogen bonds between water molecules, their working mechanisms, and the impact they have on the physical and biological properties of water.
The Importance of Hydrogen Bonds
Hydrogen bonds are a type of intermolecular force that arises between molecules with a hydrogen atom bonded to a highly electronegative atom, such as oxygen, nitrogen, or fluorine. In the case of water molecules, the oxygen atom is electronegative, which means it has a slightly negative charge. This negative charge attracts the hydrogen atoms of adjacent water molecules, creating a weak electrostatic attraction between the molecules.

The importance of hydrogen bonds between water molecules cannot be overstated. They are responsible for many of the unique properties of water, including its high surface tension, boiling point, and ability to dissolve a wide variety of substances. Hydrogen bonds also play a crucial role in the structure and function of biological molecules, such as proteins and DNA.
How Hydrogen Bonds Work
Hydrogen bonds work by creating a weak electrostatic attraction between molecules. This attraction arises between the slightly positive charge on the hydrogen atom and the slightly negative charge on the electronegative atom. The strength of the hydrogen bond depends on the distance between the molecules and the electronegativity of the atoms involved.
In the case of water molecules, the oxygen atom is electronegative, which means it has a slightly negative charge. This negative charge attracts the hydrogen atoms of adjacent water molecules, creating a weak electrostatic attraction between the molecules. The strength of this attraction is influenced by the distance between the molecules and the temperature of the system.

The Impact of Hydrogen Bonds on the Physical Properties of Water
Hydrogen bonds have a significant impact on the physical properties of water. They are responsible for many of the unique characteristics of water, including its high surface tension, boiling point, and ability to dissolve a wide variety of substances.
One of the most significant effects of hydrogen bonds on the physical properties of water is their impact on its surface tension. Surface tension is the energy that is required to increase the surface area of a liquid. In the case of water, the hydrogen bonds between molecules create a strong network of attractions that makes it difficult to increase the surface area. This results in a high surface tension, which is responsible for the ability of water to resist external forces, such as gravity.

The Impact of Hydrogen Bonds on the Biological Properties of Water
Hydrogen bonds also have a significant impact on the biological properties of water. They play a crucial role in the structure and function of biological molecules, such as proteins and DNA.
One of the most significant effects of hydrogen bonds on the biological properties of water is their impact on protein structure and function. Proteins are complex biological molecules that are composed of long chains of amino acids. The structure and function of proteins are influenced by the hydrogen bonds between the amino acids, which create a network of attractions that stabilize the protein's conformation.

FAQ Section
What are hydrogen bonds?
Hydrogen bonds are a type of intermolecular force that arises between molecules with a hydrogen atom bonded to a highly electronegative atom, such as oxygen, nitrogen, or fluorine.
How do hydrogen bonds work?
Hydrogen bonds work by creating a weak electrostatic attraction between molecules. This attraction arises between the slightly positive charge on the hydrogen atom and the slightly negative charge on the electronegative atom.
What is the impact of hydrogen bonds on the physical properties of water?
Hydrogen bonds have a significant impact on the physical properties of water, including its high surface tension, boiling point, and ability to dissolve a wide variety of substances.
What is the impact of hydrogen bonds on the biological properties of water?
Hydrogen bonds play a crucial role in the structure and function of biological molecules, such as proteins and DNA.
Conclusion
In conclusion, hydrogen bonds between water molecules are a crucial aspect of the structure and properties of water. These weak electrostatic attractions between molecules are responsible for many of the unique characteristics of water, including its high surface tension, boiling point, and ability to dissolve a wide variety of substances. Hydrogen bonds also play a crucial role in the structure and function of biological molecules, such as proteins and DNA.