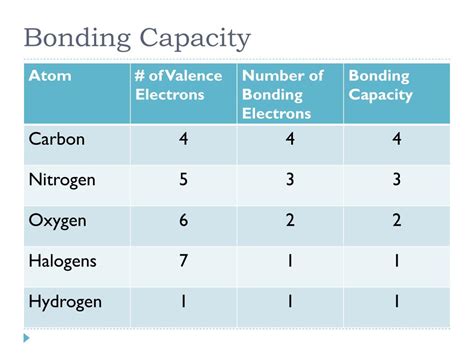
Understanding the Bonding Capacity of Elements

The bonding capacity of an element refers to its ability to form chemical bonds with other elements. This concept is crucial in understanding the structure and properties of molecules, as well as the reactions that occur between them. In this article, we will explore five ways to determine the bonding capacity of elements, which is essential in understanding the fundamental principles of chemistry.
Elements are the building blocks of matter, and their bonding capacity is a critical factor in determining their reactivity and the types of compounds they can form. By understanding the bonding capacity of elements, we can predict their behavior in different chemical reactions and environments. This knowledge is vital in various fields, including chemistry, biology, materials science, and pharmacology.
The bonding capacity of an element is determined by its electronic configuration, which is the arrangement of electrons in its atomic orbitals. The outermost energy level of an atom, also known as the valence shell, plays a crucial role in determining its bonding capacity. In this article, we will delve into the five ways to determine the bonding capacity of elements, including the octet rule, electron configuration, electronegativity, ionization energy, and the periodic table.
The Octet Rule

The octet rule states that atoms tend to gain, lose, or share electrons to achieve a full outer energy level, which typically consists of eight electrons. This rule is based on the observation that atoms with a full outer energy level are chemically stable and unreactive. The octet rule is a useful guideline for predicting the bonding capacity of elements, as it suggests that atoms will form bonds to achieve a full outer energy level.
For example, sodium (Na) has an electron configuration of 1s² 2s² 2p⁶ 3s¹, which means it has one electron in its outermost energy level. To achieve a full outer energy level, sodium will lose one electron to form a positive ion (Na⁺). This process is known as ionization, and it allows sodium to form bonds with other elements that have a full outer energy level.
Electron Configuration
The electron configuration of an element is a crucial factor in determining its bonding capacity. The electron configuration shows the arrangement of electrons in an atom's orbitals, which are the regions around the nucleus where electrons are likely to be found. By analyzing the electron configuration of an element, we can determine its bonding capacity and predict the types of bonds it can form.
For example, carbon (C) has an electron configuration of 1s² 2s² 2p², which means it has four electrons in its outermost energy level. To achieve a full outer energy level, carbon will form four bonds with other elements, such as hydrogen (H) or oxygen (O). This process is known as covalent bonding, and it allows carbon to form a wide range of compounds, including sugars, fats, and proteins.
Electronegativity

Electronegativity is a measure of an atom's ability to attract electrons in a covalent bond. It is a critical factor in determining the bonding capacity of elements, as it influences the distribution of electrons in a molecule. Electronegativity is measured on the Pauling scale, which ranges from 0 to 4.0.
For example, fluorine (F) has an electronegativity of 3.98, which means it is highly electronegative and can attract electrons strongly. This property allows fluorine to form strong bonds with other elements, such as hydrogen (H) or carbon (C). On the other hand, cesium (Cs) has an electronegativity of 0.79, which means it is weakly electronegative and can form weak bonds with other elements.
Ionization Energy
Ionization energy is the energy required to remove an electron from an atom. It is a critical factor in determining the bonding capacity of elements, as it influences the ease with which an atom can form bonds. Ionization energy is measured in electronvolts (eV), and it typically decreases as we move down a group in the periodic table.
For example, helium (He) has an ionization energy of 24.6 eV, which means it is difficult to remove an electron from a helium atom. This property makes helium unreactive and unable to form bonds with other elements. On the other hand, cesium (Cs) has an ionization energy of 3.89 eV, which means it is easy to remove an electron from a cesium atom. This property makes cesium highly reactive and able to form bonds with other elements.
The Periodic Table

The periodic table is a powerful tool for determining the bonding capacity of elements. The periodic table arranges elements in a logical and systematic way, based on their atomic number and electron configuration. By analyzing the periodic table, we can identify trends and patterns in the bonding capacity of elements.
For example, the elements in Group 1 of the periodic table, such as lithium (Li) and sodium (Na), have a strong tendency to lose electrons and form positive ions. This property makes them highly reactive and able to form bonds with other elements. On the other hand, the elements in Group 17 of the periodic table, such as fluorine (F) and chlorine (Cl), have a strong tendency to gain electrons and form negative ions. This property makes them highly reactive and able to form bonds with other elements.
By understanding the five ways to determine the bonding capacity of elements, we can gain a deeper appreciation for the complex and fascinating world of chemistry. Whether you are a student, researcher, or simply a curious individual, this knowledge can help you unlock the secrets of the periodic table and understand the behavior of elements in different chemical reactions and environments.
What is the octet rule, and how does it relate to the bonding capacity of elements?
+The octet rule states that atoms tend to gain, lose, or share electrons to achieve a full outer energy level, which typically consists of eight electrons. This rule is a useful guideline for predicting the bonding capacity of elements, as it suggests that atoms will form bonds to achieve a full outer energy level.
How does electronegativity influence the bonding capacity of elements?
+Electronegativity is a measure of an atom's ability to attract electrons in a covalent bond. It influences the distribution of electrons in a molecule and can affect the strength and type of bonds that an element can form.
What is the relationship between ionization energy and the bonding capacity of elements?
+Ionization energy is the energy required to remove an electron from an atom. It influences the ease with which an atom can form bonds and can affect the reactivity of an element.