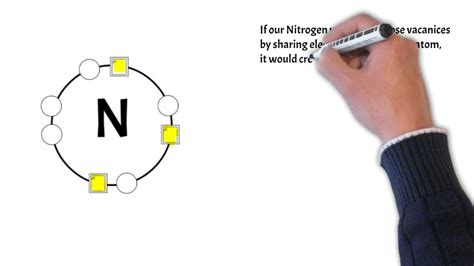
Nitrogen, a nonmetal element, plays a vital role in various biological and chemical processes due to its ability to form covalent bonds with other elements. Covalent bonds are chemical bonds that involve the sharing of electron pairs between atoms. Nitrogen's unique electron configuration allows it to form covalent bonds in multiple ways, making it a crucial component of many biomolecules and synthetic compounds.
Nitrogen's ability to form covalent bonds is largely attributed to its electron configuration. A nitrogen atom has seven electrons, with five of them occupying the outermost energy level. This leaves three unpaired electrons in the outermost energy level, allowing nitrogen to form three covalent bonds with other atoms. This unique electron configuration enables nitrogen to form covalent bonds in various ways, making it a fundamental element in many biological and chemical processes.
Nitrogen's versatility in forming covalent bonds is essential for the structure and function of biomolecules, such as amino acids, nucleotides, and lipids. In these biomolecules, nitrogen forms covalent bonds with other elements, such as carbon, oxygen, and hydrogen, to create complex molecular structures. These molecular structures are crucial for various biological processes, including protein synthesis, DNA replication, and energy metabolism.
1. Nitrogen Forms Covalent Bonds through Sigma (σ) Bonds

Nitrogen forms covalent bonds through sigma (σ) bonds, which involve the head-on overlap of atomic orbitals. In a sigma bond, the atomic orbitals of two atoms overlap along the bond axis, resulting in a symmetrical, cylindrical bond. Nitrogen forms sigma bonds with other atoms, such as carbon and hydrogen, to create stable molecular structures.
Sigma bonds are strong and stable, providing a foundation for the structure and function of biomolecules. In amino acids, for example, nitrogen forms sigma bonds with carbon and hydrogen atoms to create the amino group (-NH2). This amino group is crucial for protein synthesis, as it allows amino acids to link together to form polypeptide chains.
Example: Ammonia (NH3)
Ammonia (NH3) is a simple compound that illustrates nitrogen's ability to form sigma bonds. In ammonia, nitrogen forms three sigma bonds with hydrogen atoms, resulting in a stable, trigonal pyramidal molecular structure. This molecular structure is essential for ammonia's role in various biological processes, including protein synthesis and nitrogen metabolism.
2. Nitrogen Forms Covalent Bonds through Pi (π) Bonds

Nitrogen also forms covalent bonds through pi (π) bonds, which involve the side-by-side overlap of atomic orbitals. In a pi bond, the atomic orbitals of two atoms overlap above and below the bond axis, resulting in a nodal plane. Nitrogen forms pi bonds with other atoms, such as carbon and oxygen, to create complex molecular structures.
Pi bonds are weaker than sigma bonds but play a crucial role in the structure and function of biomolecules. In nucleotides, for example, nitrogen forms pi bonds with carbon and oxygen atoms to create the nucleobase. This nucleobase is essential for DNA replication and transcription, as it allows nucleotides to pair with each other to form the double helix structure.
Example: Pyridine (C5H5N)
Pyridine (C5H5N) is a heterocyclic compound that illustrates nitrogen's ability to form pi bonds. In pyridine, nitrogen forms two pi bonds with carbon atoms, resulting in a stable, planar molecular structure. This molecular structure is essential for pyridine's role in various biological processes, including enzyme catalysis and DNA binding.
3. Nitrogen Forms Covalent Bonds through Hydrogen Bonds

Nitrogen also forms covalent bonds through hydrogen bonds, which involve the attraction between a hydrogen atom bonded to a highly electronegative atom (such as nitrogen, oxygen, or fluorine) and another electronegative atom. Hydrogen bonds are weaker than sigma and pi bonds but play a crucial role in the structure and function of biomolecules.
Hydrogen bonds are essential for the structure and function of biomolecules, such as proteins and nucleic acids. In proteins, for example, nitrogen forms hydrogen bonds with oxygen and hydrogen atoms to create the secondary and tertiary structures. These structures are crucial for protein function, as they allow proteins to bind to specific ligands and perform specific biological processes.
Example: DNA Base Pairing
DNA base pairing is a classic example of nitrogen's ability to form hydrogen bonds. In DNA, nitrogen forms hydrogen bonds with oxygen and hydrogen atoms to create the base pairs. These base pairs are essential for DNA replication and transcription, as they allow nucleotides to pair with each other to form the double helix structure.
What is the electron configuration of nitrogen?
+Nitrogen has seven electrons, with five of them occupying the outermost energy level. This leaves three unpaired electrons in the outermost energy level, allowing nitrogen to form three covalent bonds with other atoms.
What is the difference between sigma (σ) bonds and pi (π) bonds?
+Sigma (σ) bonds involve the head-on overlap of atomic orbitals, while pi (π) bonds involve the side-by-side overlap of atomic orbitals. Sigma bonds are stronger and more stable than pi bonds.
What is the role of nitrogen in biomolecules?
+Nitrogen plays a crucial role in the structure and function of biomolecules, such as amino acids, nucleotides, and lipids. Nitrogen forms covalent bonds with other elements, such as carbon, oxygen, and hydrogen, to create complex molecular structures.
In conclusion, nitrogen's ability to form covalent bonds is essential for the structure and function of biomolecules. Nitrogen forms covalent bonds through sigma (σ) bonds, pi (π) bonds, and hydrogen bonds, making it a fundamental element in various biological processes. Understanding nitrogen's covalent bonding mechanisms is crucial for appreciating its role in biomolecules and its importance in various biological processes.