Oxygen is one of the most essential elements in the universe, and its bonding capacity plays a crucial role in the formation of various molecules that sustain life. In this article, we will delve into the world of oxygen's bonding capacity, exploring how many bonds it forms and the significance of these bonds in different contexts.
Oxygen's Bonding Capacity: An Overview

Oxygen is a highly reactive element that readily forms bonds with other atoms. Its high electronegativity and ability to form multiple bonds make it an excellent bonding partner. Oxygen's bonding capacity is primarily due to its electronic configuration, which consists of six valence electrons in its outermost energy level. This configuration allows oxygen to form a maximum of four bonds with other atoms, either through covalent or ionic bonding mechanisms.
Covalent Bonds: Oxygen's Primary Bonding Mechanism
Covalent bonds are the primary mechanism by which oxygen forms bonds with other atoms. In a covalent bond, oxygen shares its valence electrons with other atoms to achieve a stable electronic configuration. This sharing of electrons results in the formation of a strong chemical bond between the oxygen atom and the other atom.
Oxygen can form covalent bonds with various atoms, including hydrogen, carbon, nitrogen, and sulfur. These bonds are essential in the formation of biomolecules, such as carbohydrates, proteins, and nucleic acids. For example, the covalent bond between oxygen and hydrogen atoms forms water (H2O), which is essential for life.
How Many Bonds Does Oxygen Form?

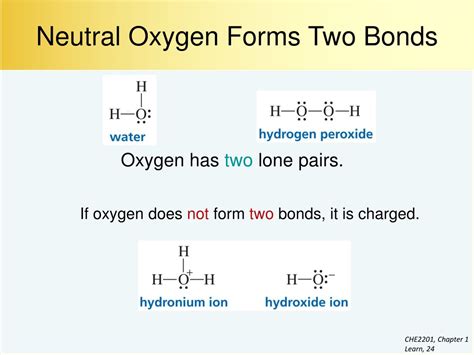
Oxygen can form a maximum of four bonds with other atoms. This is due to its electronic configuration, which consists of six valence electrons in its outermost energy level. When oxygen forms bonds with other atoms, it shares its valence electrons to achieve a stable electronic configuration.
In general, oxygen forms two or three bonds with other atoms. The number of bonds formed depends on the specific molecule and the atoms involved. For example, in water (H2O), oxygen forms two covalent bonds with hydrogen atoms. In carbon dioxide (CO2), oxygen forms two covalent bonds with carbon atoms.
Oxygen's Bonding Capacity in Biomolecules
Oxygen's bonding capacity plays a crucial role in the formation of biomolecules. Biomolecules are essential for life, and oxygen's ability to form multiple bonds makes it an excellent bonding partner.
In carbohydrates, oxygen forms covalent bonds with carbon and hydrogen atoms. These bonds are essential in the formation of sugars, starches, and fibers. In proteins, oxygen forms covalent bonds with nitrogen and hydrogen atoms. These bonds are essential in the formation of amino acids, which are the building blocks of proteins.
Oxygen's Bonding Capacity in the Atmosphere

Oxygen's bonding capacity also plays a crucial role in the atmosphere. In the atmosphere, oxygen forms bonds with other gases, such as nitrogen and carbon dioxide. These bonds are essential in the formation of the ozone layer, which protects life on Earth from harmful ultraviolet radiation.
In addition, oxygen's bonding capacity is essential in the formation of clouds and precipitation. Oxygen forms bonds with water vapor in the atmosphere, resulting in the formation of clouds and precipitation.
Importance of Oxygen's Bonding Capacity
Oxygen's bonding capacity is essential for life on Earth. Its ability to form multiple bonds makes it an excellent bonding partner, and its high electronegativity makes it highly reactive.
Oxygen's bonding capacity is essential in the formation of biomolecules, such as carbohydrates, proteins, and nucleic acids. These biomolecules are essential for life, and oxygen's ability to form multiple bonds makes it an excellent bonding partner.
In addition, oxygen's bonding capacity is essential in the atmosphere. Its ability to form bonds with other gases, such as nitrogen and carbon dioxide, is essential in the formation of the ozone layer and clouds.
Conclusion: Oxygen's Bonding Capacity

In conclusion, oxygen's bonding capacity is a critical aspect of its chemistry. Its ability to form multiple bonds makes it an excellent bonding partner, and its high electronegativity makes it highly reactive.
Oxygen's bonding capacity is essential in the formation of biomolecules, such as carbohydrates, proteins, and nucleic acids. Its ability to form bonds with other gases, such as nitrogen and carbon dioxide, is essential in the formation of the ozone layer and clouds.
We hope this article has provided you with a comprehensive understanding of oxygen's bonding capacity. If you have any questions or comments, please feel free to share them below.
What is oxygen's bonding capacity?
+Oxygen's bonding capacity refers to its ability to form multiple bonds with other atoms. Oxygen can form a maximum of four bonds with other atoms, making it an excellent bonding partner.
Why is oxygen's bonding capacity important?
+Oxygen's bonding capacity is essential for life on Earth. Its ability to form multiple bonds makes it an excellent bonding partner, and its high electronegativity makes it highly reactive. Oxygen's bonding capacity is essential in the formation of biomolecules, such as carbohydrates, proteins, and nucleic acids.
How many bonds can oxygen form?
+Oxygen can form a maximum of four bonds with other atoms. However, in general, oxygen forms two or three bonds with other atoms, depending on the specific molecule and the atoms involved.