Magnesium is an essential mineral that plays a crucial role in various bodily functions, including muscle and nerve function, energy production, and bone health. It is the second most abundant element in cells and the fourth most abundant mineral in the body. Magnesium can form two types of bonds: ionic bonds and covalent bonds. In this article, we will explore the two bonds magnesium can form and their significance in biological systems.
Understanding Magnesium Chemistry

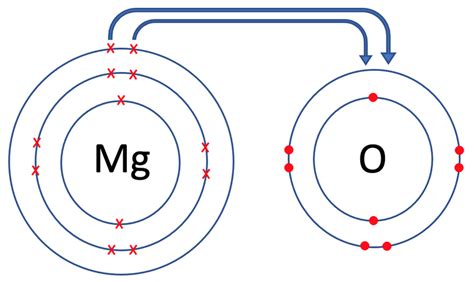
Magnesium is a highly reactive metal that readily loses two electrons to form a positively charged ion, Mg2+. This property allows magnesium to form bonds with other elements. The two main types of bonds magnesium can form are ionic bonds and covalent bonds.
What are Ionic Bonds?
Ionic bonds are formed when magnesium loses two electrons to form a positively charged ion, which then attracts a negatively charged ion, typically a nonmetal such as oxygen or chlorine. This attraction between oppositely charged ions results in the formation of an ionic bond. Ionic bonds are strong and rigid, holding the ions together in a crystalline lattice structure.
What are Covalent Bonds?
Covalent bonds, on the other hand, are formed when magnesium shares one or more pairs of electrons with another element, typically a nonmetal such as oxygen or nitrogen. This sharing of electrons results in the formation of a covalent bond. Covalent bonds are typically weaker than ionic bonds and are more flexible.
Magnesium Ionic Bonds

Magnesium ionic bonds are essential in various biological processes, including:
- Bone health: Magnesium ions are crucial for the formation and maintenance of bone tissue. They help regulate the activity of osteoblasts and osteoclasts, cells responsible for bone growth and resorption.
- Neurotransmission: Magnesium ions play a critical role in neurotransmission, regulating the release of neurotransmitters such as acetylcholine and dopamine.
- Muscle function: Magnesium ions help regulate muscle contraction and relaxation, essential for muscle function and movement.
Examples of magnesium ionic bonds include:
- Magnesium oxide (MgO): A compound formed by the reaction of magnesium and oxygen.
- Magnesium chloride (MgCl2): A compound formed by the reaction of magnesium and chlorine.
Magnesium Covalent Bonds

Magnesium covalent bonds are also essential in various biological processes, including:
- Enzyme function: Magnesium ions are required for the activity of many enzymes, including those involved in energy production and DNA synthesis.
- Protein structure: Magnesium ions help stabilize the structure of proteins, including enzymes and hormones.
- Cell signaling: Magnesium ions play a critical role in cell signaling, regulating the activity of proteins and enzymes involved in signal transduction pathways.
Examples of magnesium covalent bonds include:
- Magnesium ATP (Mg-ATP): A compound formed by the reaction of magnesium and ATP, essential for energy production.
- Magnesium acetyl-CoA (Mg-acetyl-CoA): A compound formed by the reaction of magnesium and acetyl-CoA, essential for fatty acid synthesis.
Importance of Magnesium Bonds in Biological Systems

Magnesium bonds, both ionic and covalent, play a crucial role in various biological processes. They are essential for maintaining proper cellular function, regulating enzyme activity, and supporting overall health. Magnesium deficiency can lead to a range of health problems, including muscle cramps, weakness, and fatigue.
In conclusion, magnesium can form two types of bonds: ionic bonds and covalent bonds. These bonds are essential for various biological processes, including bone health, neurotransmission, muscle function, enzyme function, protein structure, and cell signaling. Understanding the importance of magnesium bonds in biological systems can help us appreciate the critical role this mineral plays in maintaining overall health.
We invite you to share your thoughts on the importance of magnesium bonds in biological systems. Do you have any questions or comments on this topic? Please leave them in the comment section below.
What is the main difference between ionic and covalent bonds?
+The main difference between ionic and covalent bonds is the way electrons are shared between atoms. Ionic bonds are formed when one atom loses or gains electrons to form ions, which then attract each other. Covalent bonds are formed when atoms share one or more pairs of electrons.
What are some examples of magnesium ionic bonds?
+Examples of magnesium ionic bonds include magnesium oxide (MgO) and magnesium chloride (MgCl2).
What are some examples of magnesium covalent bonds?
+Examples of magnesium covalent bonds include magnesium ATP (Mg-ATP) and magnesium acetyl-CoA (Mg-acetyl-CoA).