Beryllium, the fourth element in the periodic table, has been a topic of interest in the scientific community due to its unique properties and potential applications. One of the most frequently asked questions about beryllium is how many bonds it can form. In this article, we will delve into the world of beryllium chemistry and explore the answer to this question.
Understanding Beryllium's Electronic Configuration

To understand how many bonds beryllium can form, we need to look at its electronic configuration. Beryllium has an atomic number of 4 and an atomic mass of 9.0122 g/mol. Its electronic configuration is 1s² 2s², which means it has two electrons in its outermost energy level. This configuration plays a crucial role in determining the number of bonds beryllium can form.
What is a Bond?
Before we dive into the specifics of beryllium bonding, let's take a step back and define what a bond is. In chemistry, a bond is a chemical link between two atoms that holds them together. Bonds are formed when atoms share or exchange electrons to achieve a stable electronic configuration. There are several types of bonds, including covalent, ionic, and metallic bonds.
How Many Bonds Can Beryllium Form?

Beryllium can form a maximum of four bonds. This is because it has four valence electrons, which are the electrons in its outermost energy level. These electrons can be shared with other atoms to form covalent bonds. Beryllium's ability to form four bonds makes it a versatile element that can form a wide range of compounds.
Why Can Beryllium Form Four Bonds?
Beryllium's ability to form four bonds can be attributed to its electronic configuration. As mentioned earlier, beryllium has two electrons in its outermost energy level. These electrons are in the 2s orbital, which is a spherical orbital that can hold a maximum of two electrons. However, beryllium can also promote its 2s electrons to the 2p orbital, which can hold a maximum of six electrons. This promotion allows beryllium to form four bonds.
Types of Bonds Formed by Beryllium

Beryllium can form several types of bonds, including:
- Covalent bonds: Beryllium can form covalent bonds with other nonmetals, such as oxygen, nitrogen, and carbon. These bonds are formed by sharing electrons between atoms.
- Ionic bonds: Beryllium can also form ionic bonds with other elements, such as halogens. These bonds are formed by transferring electrons between atoms.
- Metallic bonds: Beryllium can form metallic bonds with other metals, such as copper and aluminum. These bonds are formed by sharing electrons among atoms.
Examples of Beryllium Compounds
Beryllium forms a wide range of compounds, including:
- Beryllium oxide (BeO): This compound is formed by reacting beryllium with oxygen.
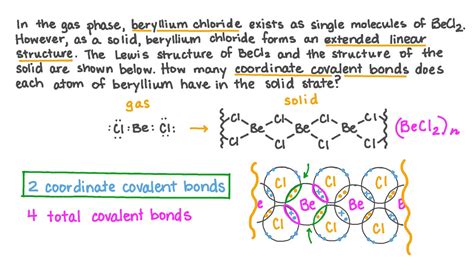
- Beryllium chloride (BeCl₂): This compound is formed by reacting beryllium with chlorine.
- Beryllium sulfate (BeSO₄): This compound is formed by reacting beryllium with sulfuric acid.
Importance of Beryllium Bonds

Beryllium bonds are important in various applications, including:
- Aerospace industry: Beryllium is used in the aerospace industry due to its high strength-to-weight ratio and resistance to corrosion.
- Nuclear industry: Beryllium is used in nuclear reactors due to its ability to absorb neutrons.
- Electronics industry: Beryllium is used in electronic components, such as springs and contacts, due to its high conductivity and resistance to corrosion.
Conclusion
In conclusion, beryllium can form a maximum of four bonds. Its electronic configuration and ability to promote its 2s electrons to the 2p orbital allow it to form a wide range of compounds. Beryllium bonds are important in various applications, including the aerospace, nuclear, and electronics industries.
What is the electronic configuration of beryllium?
+Beryllium has an electronic configuration of 1s² 2s².
How many bonds can beryllium form?
+Beryllium can form a maximum of four bonds.
What are the types of bonds formed by beryllium?
+Beryllium can form covalent, ionic, and metallic bonds.