The discovery of the structure of nucleic acids is one of the most significant scientific breakthroughs of the 20th century. At the heart of this discovery lies the unique bonding mechanism between nucleotides, which forms the backbone of nucleic acids. Understanding the intricacies of nucleotides bonding is essential to grasping the fundamental principles of genetics, molecular biology, and the very essence of life itself.

Nucleic acids, including DNA (deoxyribonucleic acid) and RNA (ribonucleic acid), are the blueprints of life, containing the genetic instructions necessary for the development, growth, and function of all living organisms. The backbone of nucleic acids is composed of nucleotides, which are linked together through a process known as phosphodiester bonding.
What are Nucleotides?
A nucleotide is a molecule composed of three main components: a nitrogenous base, a sugar molecule, and a phosphate group. The nitrogenous base is a heterocyclic compound that contains nitrogen and carbon atoms. There are five different nitrogenous bases found in nucleic acids: adenine (A), guanine (G), cytosine (C), thymine (T), and uracil (U). The sugar molecule is either deoxyribose (in DNA) or ribose (in RNA), while the phosphate group is a phosphoric acid residue.

The Phosphodiester Bonding Mechanism
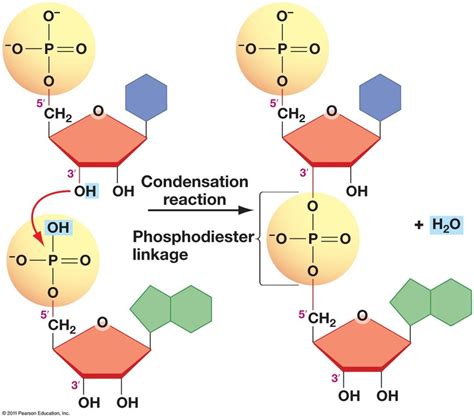
The phosphodiester bond is a covalent bond between the phosphate group of one nucleotide and the sugar molecule of another. This bond forms the backbone of nucleic acids, linking nucleotides together in a chain-like structure. The phosphodiester bond is a condensation reaction, where the phosphate group of one nucleotide reacts with the hydroxyl group of the sugar molecule of another, releasing a water molecule in the process.
The phosphodiester bond is a strong and stable bond, allowing the nucleic acid molecule to maintain its structure and function. However, it is not a rigid bond, allowing for some degree of flexibility and rotation. This flexibility is essential for the replication and transcription of nucleic acids, as it allows the molecule to be unwound and accessed by enzymes.
The Formation of the Phosphodiester Bond
The formation of the phosphodiester bond is a complex process that involves a series of enzyme-catalyzed reactions. The process begins with the activation of the phosphate group of one nucleotide, followed by the binding of the sugar molecule of another nucleotide to the enzyme. The enzyme then catalyzes the condensation reaction between the phosphate group and the sugar molecule, releasing a water molecule in the process.
The phosphodiester bond is formed through a process known as nucleophilic attack, where the hydroxyl group of the sugar molecule attacks the phosphate group of the adjacent nucleotide. This reaction is catalyzed by enzymes known as DNA or RNA polymerases, which are responsible for the replication and transcription of nucleic acids.

Base Pairing and the Double Helix Structure
The unique structure of nucleic acids is also characterized by the formation of base pairs between complementary nitrogenous bases. Adenine (A) pairs with thymine (T) in DNA, while guanine (G) pairs with cytosine (C). These base pairs are stabilized by hydrogen bonds, which hold the two strands of the nucleic acid molecule together in a double helix structure.
The double helix structure of nucleic acids is essential for their function and stability. The sugar-phosphate backbone of the nucleic acid molecule is stabilized by the stacking of the base pairs, which provides a rigid framework for the molecule. The double helix structure also allows for the replication and transcription of nucleic acids, as it provides a template for the synthesis of new nucleic acid molecules.

Conclusion
In conclusion, the bonding mechanism between nucleotides is a fundamental aspect of the structure and function of nucleic acids. The phosphodiester bond is a strong and stable bond that forms the backbone of nucleic acids, linking nucleotides together in a chain-like structure. The unique structure of nucleic acids, characterized by the formation of base pairs and the double helix structure, is essential for their function and stability.
As we continue to explore the intricacies of nucleic acids and their role in the biological world, we are reminded of the importance of understanding the fundamental principles of genetics and molecular biology. The discovery of the structure of nucleic acids is a testament to the power of scientific inquiry and the importance of continued research in the life sciences.
Share Your Thoughts!
We hope this article has provided you with a deeper understanding of the bonding mechanism between nucleotides and the structure of nucleic acids. Share your thoughts and questions in the comments below! Do you have any experiences or insights related to the topic? Share them with us!
What is the role of nucleotides in the structure of nucleic acids?
+Nucleotides are the building blocks of nucleic acids, providing the sugar-phosphate backbone and the nitrogenous bases that pair to form the genetic code.
What is the phosphodiester bond, and how is it formed?
+The phosphodiester bond is a covalent bond between the phosphate group of one nucleotide and the sugar molecule of another. It is formed through a condensation reaction catalyzed by enzymes known as DNA or RNA polymerases.
What is the significance of the double helix structure of nucleic acids?
+The double helix structure of nucleic acids provides a stable framework for the genetic code, allowing for the replication and transcription of nucleic acids. It is essential for the function and stability of nucleic acids.