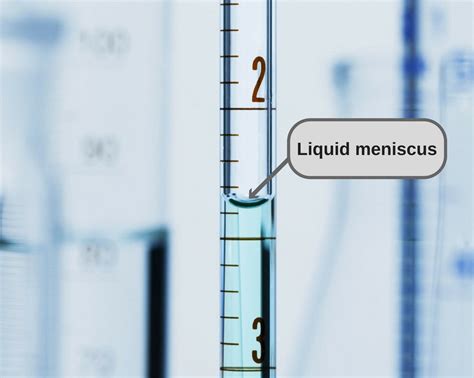
Have you ever filled a test tube with a liquid and noticed that the surface of the liquid curves upward or downward, forming a concave or convex shape? This phenomenon is known as the meniscus, and it's a fascinating aspect of fluid dynamics that has puzzled scientists and non-scientists alike for centuries. In this article, we'll delve into the world of menisci, exploring the reasons behind this curious behavior and its significance in various scientific and everyday applications.
The meniscus is a fundamental concept in physics and chemistry, and understanding its underlying mechanisms can provide valuable insights into the behavior of liquids in various environments. From the shape of a liquid's surface in a test tube to the wettability of surfaces, the meniscus plays a crucial role in determining the interactions between liquids and their containers. In this article, we'll examine the science behind the meniscus, its characteristics, and its applications in various fields.

What is a Meniscus?
A meniscus is the curved surface of a liquid in a container, such as a test tube or a glass. The meniscus can be either concave or convex, depending on the properties of the liquid and the material of the container. In general, a concave meniscus is formed when the liquid wets the container, while a convex meniscus is formed when the liquid does not wet the container.
The shape of the meniscus is determined by the balance between two main forces: adhesion and cohesion. Adhesion is the force of attraction between the liquid and the container, while cohesion is the force of attraction between the liquid molecules themselves. When the adhesion force is stronger than the cohesion force, the liquid wets the container, forming a concave meniscus. Conversely, when the cohesion force is stronger, the liquid does not wet the container, forming a convex meniscus.
Types of Menisci
There are two main types of menisci: concave and convex.
- Concave Meniscus: A concave meniscus is formed when the liquid wets the container. This type of meniscus is commonly observed in liquids with high surface tension, such as water and mercury. In a concave meniscus, the liquid surface is curved downward, forming a U-shaped profile.
- Convex Meniscus: A convex meniscus is formed when the liquid does not wet the container. This type of meniscus is commonly observed in liquids with low surface tension, such as ethanol and gasoline. In a convex meniscus, the liquid surface is curved upward, forming a dome-shaped profile.


Causes of Meniscus
The meniscus is caused by the interplay between adhesion and cohesion forces. The shape of the meniscus is determined by the balance between these two forces. In general, the meniscus is influenced by the following factors:
- Surface Tension: The surface tension of a liquid is a measure of the force that acts along the surface of the liquid, causing it to behave as if it has an "elastic skin" at its surface. Liquids with high surface tension, such as water, tend to form concave menisci, while liquids with low surface tension, such as ethanol, tend to form convex menisci.
- Wettability: The wettability of a surface is a measure of its ability to attract and hold onto a liquid. Surfaces with high wettability, such as glass and ceramics, tend to form concave menisci, while surfaces with low wettability, such as plastics and metals, tend to form convex menisci.
- Temperature: Temperature can affect the shape of the meniscus by changing the surface tension and wettability of the liquid. In general, increasing the temperature of a liquid tends to decrease its surface tension, leading to a convex meniscus.
Effects of Meniscus
The meniscus can have significant effects on various scientific and everyday applications. Some of the effects of meniscus include:
- Measurement Errors: The meniscus can cause measurement errors when using a test tube or a graduated cylinder to measure the volume of a liquid. The curved surface of the liquid can make it difficult to read the meniscus accurately, leading to errors in measurement.
- Surface Tension: The meniscus is a manifestation of the surface tension of a liquid. Surface tension plays a crucial role in various scientific and everyday applications, such as the formation of droplets, the behavior of liquids in narrow tubes, and the wetting of surfaces.
- Wettability: The meniscus is also related to the wettability of surfaces. Wettability is an important factor in various applications, such as coating, painting, and printing.

Applications of Meniscus
The meniscus has various applications in scientific and everyday fields. Some of the applications of meniscus include:
- Laboratory Applications: The meniscus is an essential concept in laboratory settings, where it is used to measure the volume of liquids accurately. Understanding the meniscus is crucial in various laboratory applications, such as titration, chromatography, and spectroscopy.
- Coating and Painting: The meniscus is related to the wettability of surfaces, which is an important factor in coating and painting applications. Understanding the meniscus can help improve the quality of coatings and paints by optimizing the wettability of surfaces.
- Printing: The meniscus is also related to the wettability of surfaces, which is an important factor in printing applications. Understanding the meniscus can help improve the quality of prints by optimizing the wettability of surfaces.
Meniscus in Everyday Life
The meniscus is not just a laboratory phenomenon; it can also be observed in everyday life. Some examples of meniscus in everyday life include:
- Water in a Glass: The meniscus can be observed when pouring water into a glass. The curved surface of the water is a manifestation of the meniscus.
- Wetting of Surfaces: The meniscus is related to the wettability of surfaces, which is an important factor in various everyday applications, such as cleaning and painting.

We hope this article has helped you understand the fascinating world of menisci. Whether you're a scientist, a student, or simply someone curious about the natural world, the meniscus is an intriguing phenomenon that can provide valuable insights into the behavior of liquids and surfaces. So next time you pour a liquid into a container or observe the wettability of a surface, remember the meniscus and the important role it plays in our daily lives.
If you have any questions or comments about this article, please feel free to share them with us. We'd love to hear from you and explore the world of menisci further.
What is the difference between a concave and convex meniscus?
+A concave meniscus is formed when the liquid wets the container, while a convex meniscus is formed when the liquid does not wet the container.
What are the causes of meniscus?
+The meniscus is caused by the interplay between adhesion and cohesion forces. The shape of the meniscus is determined by the balance between these two forces.
What are the effects of meniscus?
+The meniscus can have significant effects on various scientific and everyday applications, such as measurement errors, surface tension, and wettability.