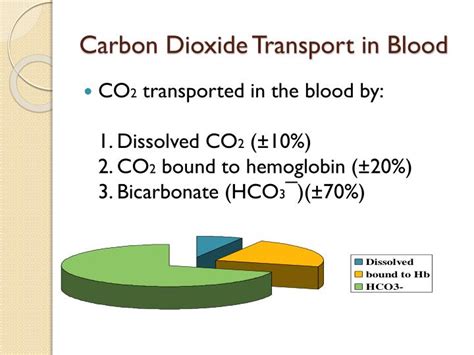
Carbon dioxide (CO2) is a byproduct of cellular metabolism that must be transported from the tissues to the lungs for exhalation. The transportation of CO2 in the blood is a complex process that involves multiple mechanisms. In this article, we will explore the three main ways CO2 is transported in the blood.
Introduction to CO2 Transport in the Blood

The transportation of CO2 in the blood is a critical function that helps maintain acid-base balance in the body. CO2 is produced by the cells as a waste product of metabolism and must be removed from the body to prevent acidosis. The blood plays a crucial role in transporting CO2 from the tissues to the lungs, where it is exhaled.
Mechanisms of CO2 Transport in the Blood

There are three main mechanisms by which CO2 is transported in the blood:
1. Dissolved in Plasma
CO2 can dissolve directly in the plasma, where it reacts with water to form carbonic acid. This reaction is catalyzed by the enzyme carbonic anhydrase, which is present in red blood cells. The carbonic acid then dissociates into hydrogen ions and bicarbonate ions. This mechanism accounts for about 5-10% of CO2 transport in the blood.
2. Bound to Hemoglobin

CO2 can bind to hemoglobin, the protein in red blood cells that carries oxygen. When CO2 binds to hemoglobin, it forms carbaminohemoglobin. This reaction helps to reduce the amount of CO2 in the plasma and prevents acidosis. This mechanism accounts for about 70-80% of CO2 transport in the blood.
3. As Bicarbonate Ions
CO2 can also be transported as bicarbonate ions, which are produced when carbonic acid dissociates. Bicarbonate ions are present in both the plasma and red blood cells. In the lungs, the bicarbonate ions are converted back into CO2, which is then exhaled. This mechanism accounts for about 10-20% of CO2 transport in the blood.
Importance of CO2 Transport in the Blood

The transportation of CO2 in the blood is critical for maintaining acid-base balance in the body. If CO2 is not removed from the body, it can lead to acidosis, a condition in which the blood becomes too acidic. Acidosis can have serious consequences, including respiratory failure, cardiac arrest, and even death.
Clinical Significance of CO2 Transport in the Blood

The transportation of CO2 in the blood has important clinical implications. For example, in patients with respiratory failure, the ability to transport CO2 is impaired, leading to acidosis. In patients with kidney disease, the ability to transport CO2 is also impaired, leading to acidosis.
Conclusion
In conclusion, the transportation of CO2 in the blood is a complex process that involves multiple mechanisms. Understanding these mechanisms is critical for maintaining acid-base balance in the body and preventing acidosis.
What is the main mechanism of CO2 transport in the blood?
+The main mechanism of CO2 transport in the blood is binding to hemoglobin, which accounts for about 70-80% of CO2 transport.
What is the clinical significance of CO2 transport in the blood?
+The clinical significance of CO2 transport in the blood is critical for maintaining acid-base balance in the body and preventing acidosis, particularly in patients with respiratory failure or kidney disease.
How does CO2 transport in the blood affect acid-base balance?
+CO2 transport in the blood helps to maintain acid-base balance by removing excess CO2 from the body, which can lead to acidosis if not removed.