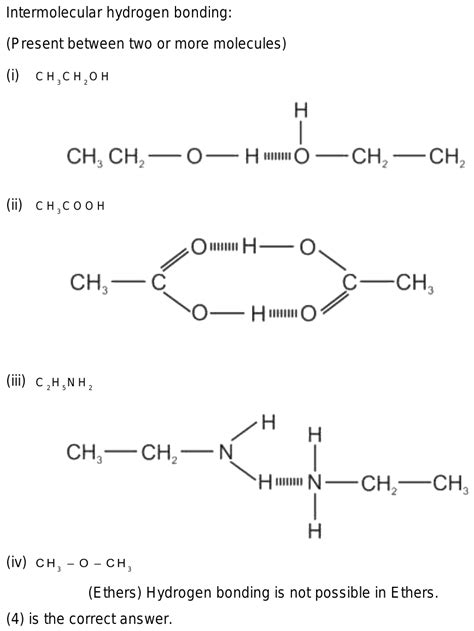
Ch3och3, also known as dimethyl ether, is a polar aprotic solvent that plays a crucial role in various chemical reactions. One of its unique properties is its ability to form hydrogen bonds, which are essential for many biological and chemical processes. In this article, we will explore three ways Ch3och3 forms hydrogen bonds and their significance in different applications.
Understanding Hydrogen Bonding in Ch3och3

Hydrogen bonding is a type of intermolecular force that arises between molecules with a hydrogen atom bonded to a highly electronegative atom, such as oxygen, nitrogen, or fluorine. In the case of Ch3och3, the oxygen atom is the electronegative atom that participates in hydrogen bonding. The methyl groups (CH3) in Ch3och3 are electron-donating, which increases the electron density around the oxygen atom, making it more susceptible to forming hydrogen bonds.
Mechanism of Hydrogen Bond Formation in Ch3och3
The mechanism of hydrogen bond formation in Ch3och3 involves the interaction between the lone pair of electrons on the oxygen atom and the partially positive hydrogen atom of another molecule. This interaction is facilitated by the electronegative oxygen atom, which pulls the shared electrons closer to itself, creating a partial positive charge on the hydrogen atom.
Way 1: Hydrogen Bonding with Water Molecules

Ch3och3 forms hydrogen bonds with water molecules, which is essential for its solubility in water. The oxygen atom in Ch3och3 acts as a hydrogen bond acceptor, while the hydrogen atoms in water molecules act as hydrogen bond donors. This interaction enables Ch3och3 to dissolve in water, making it a useful solvent in various chemical reactions.
Significance of Hydrogen Bonding with Water Molecules
The ability of Ch3och3 to form hydrogen bonds with water molecules has significant implications in various applications, including:
- Enhanced solubility: Hydrogen bonding with water molecules enables Ch3och3 to dissolve in water, making it a useful solvent in various chemical reactions.
- Biological processes: Hydrogen bonding with water molecules plays a crucial role in various biological processes, such as protein folding and enzyme catalysis.
- Pharmaceutical applications: Ch3och3 is used as a solvent in the production of various pharmaceuticals, where its ability to form hydrogen bonds with water molecules is essential for its solubility and stability.
Way 2: Hydrogen Bonding with Other Polar Molecules

Ch3och3 also forms hydrogen bonds with other polar molecules, such as alcohols, amines, and carboxylic acids. The oxygen atom in Ch3och3 acts as a hydrogen bond acceptor, while the hydrogen atoms in the polar molecules act as hydrogen bond donors. This interaction enables Ch3och3 to participate in various chemical reactions, such as nucleophilic substitution and elimination reactions.
Significance of Hydrogen Bonding with Other Polar Molecules
The ability of Ch3och3 to form hydrogen bonds with other polar molecules has significant implications in various applications, including:
- Enhanced reactivity: Hydrogen bonding with other polar molecules enables Ch3och3 to participate in various chemical reactions, such as nucleophilic substitution and elimination reactions.
- Solvent properties: Ch3och3's ability to form hydrogen bonds with other polar molecules makes it a useful solvent in various chemical reactions.
- Biological processes: Hydrogen bonding with other polar molecules plays a crucial role in various biological processes, such as protein-ligand interactions and enzyme catalysis.
Way 3: Hydrogen Bonding with Metal Ions

Ch3och3 also forms hydrogen bonds with metal ions, such as sodium and potassium ions. The oxygen atom in Ch3och3 acts as a hydrogen bond acceptor, while the metal ions act as hydrogen bond donors. This interaction enables Ch3och3 to participate in various chemical reactions, such as complexation reactions and catalytic reactions.
Significance of Hydrogen Bonding with Metal Ions
The ability of Ch3och3 to form hydrogen bonds with metal ions has significant implications in various applications, including:
- Enhanced reactivity: Hydrogen bonding with metal ions enables Ch3och3 to participate in various chemical reactions, such as complexation reactions and catalytic reactions.
- Solvent properties: Ch3och3's ability to form hydrogen bonds with metal ions makes it a useful solvent in various chemical reactions.
- Biological processes: Hydrogen bonding with metal ions plays a crucial role in various biological processes, such as enzyme catalysis and protein-ligand interactions.
What is the significance of hydrogen bonding in Ch3och3?
+Hydrogen bonding in Ch3och3 is significant because it enables the molecule to form interactions with other molecules, such as water molecules, polar molecules, and metal ions. These interactions are essential for various biological and chemical processes, including solubility, reactivity, and enzyme catalysis.
How does Ch3och3 form hydrogen bonds with water molecules?
+Ch3och3 forms hydrogen bonds with water molecules through the interaction between the lone pair of electrons on the oxygen atom and the partially positive hydrogen atom of the water molecule.
What are the implications of hydrogen bonding in Ch3och3 for pharmaceutical applications?
+Hydrogen bonding in Ch3och3 has significant implications for pharmaceutical applications, including enhanced solubility and stability of pharmaceuticals. Ch3och3 is used as a solvent in the production of various pharmaceuticals, where its ability to form hydrogen bonds with water molecules is essential for its solubility and stability.
We hope this article has provided you with a comprehensive understanding of the ways Ch3och3 forms hydrogen bonds and their significance in various applications. If you have any further questions or would like to share your thoughts, please feel free to comment below.