The Periodic Table of Elements is a tabular display of the known chemical elements, organized by their atomic number, electron configuration, and recurring chemical properties. The elements are listed in order of increasing atomic number (number of protons in the nucleus) and are grouped into rows called periods and columns called groups or families. One of the most essential elements in the periodic table is Calcium (Ca), with an atomic number of 20.
What is Calcium Electron Configuration Long Form?

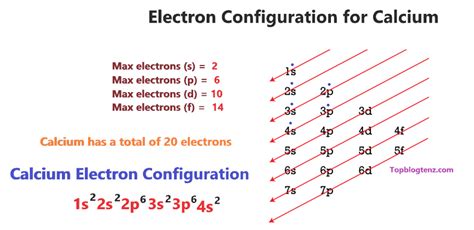
Calcium electron configuration long form is a detailed representation of the arrangement of electrons in a calcium atom. In atomic physics and chemistry, the electron configuration is the distribution of electrons of an atom or molecule in atomic or molecular orbitals. The calcium electron configuration long form is expressed as 1s² 2s² 2p⁶ 3s² 3p⁶ 4s². This notation indicates the energy levels and orbitals in which the electrons are arranged.
Understanding the Electron Configuration Notation
The electron configuration notation is a shorthand way of describing the arrangement of electrons in an atom. The notation consists of a series of numbers and letters, each representing a specific energy level and orbital. The numbers represent the energy level, and the letters represent the orbital type (s, p, d, or f).
Calcium Electron Configuration Long Form Breakdown

The calcium electron configuration long form can be broken down into several parts:
- 1s²: This represents the first energy level, which contains two electrons in the s-orbital.
- 2s²: This represents the second energy level, which contains two electrons in the s-orbital.
- 2p⁶: This represents the second energy level, which contains six electrons in the p-orbitals.
- 3s²: This represents the third energy level, which contains two electrons in the s-orbital.
- 3p⁶: This represents the third energy level, which contains six electrons in the p-orbitals.
- 4s²: This represents the fourth energy level, which contains two electrons in the s-orbital.
Significance of Calcium Electron Configuration Long Form
The calcium electron configuration long form is essential in understanding the chemical properties of calcium. The arrangement of electrons in an atom determines its chemical behavior, and the electron configuration notation provides a concise way of describing this arrangement.
Calcium Electron Configuration Long Form and Chemical Properties

The calcium electron configuration long form is closely related to its chemical properties. Calcium is a highly reactive metal, and its electron configuration plays a crucial role in its reactivity. The two electrons in the 4s-orbital are easily lost, making calcium a good reducing agent. The electron configuration notation helps predict the chemical behavior of calcium and its compounds.
Calcium Compounds and Electron Configuration
Calcium forms a wide range of compounds, including calcium oxide, calcium carbonate, and calcium chloride. The electron configuration of calcium plays a crucial role in the formation of these compounds. The electron configuration notation helps predict the chemical properties of these compounds and their reactivity.
Calcium Electron Configuration Long Form and Biological Importance

Calcium is an essential element in living organisms, and its electron configuration plays a crucial role in its biological importance. Calcium ions are involved in many biological processes, including muscle contraction, nerve transmission, and bone formation. The electron configuration notation helps understand the chemical properties of calcium ions and their role in biological processes.
Calcium Ions and Electron Configuration
Calcium ions have a positive charge, and their electron configuration is different from that of neutral calcium atoms. The electron configuration notation helps predict the chemical properties of calcium ions and their role in biological processes.
Conclusion and Future Directions
In conclusion, the calcium electron configuration long form is a detailed representation of the arrangement of electrons in a calcium atom. The notation is essential in understanding the chemical properties of calcium and its compounds. The electron configuration notation helps predict the chemical behavior of calcium and its role in biological processes.
The study of electron configuration is a rapidly evolving field, and new research is focused on understanding the electronic structure of atoms and molecules. Future directions in this field may include the development of new notation systems and the application of electron configuration to emerging fields such as nanotechnology and biotechnology.
What is the electron configuration of calcium?
+The electron configuration of calcium is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s².
What is the significance of electron configuration notation?
+The electron configuration notation is essential in understanding the chemical properties of atoms and molecules.
What is the role of calcium ions in biological processes?
+Calcium ions are involved in many biological processes, including muscle contraction, nerve transmission, and bone formation.