Argon is a noble gas that belongs to the group 18 of the periodic table. It is a chemically inert element, meaning it does not readily react with other elements to form compounds. However, under certain conditions, argon can exhibit some degree of reactivity, particularly when it comes to bonding with other elements. In this article, we will explore argon's bonding tendency with other elements and discuss the various factors that influence its reactivity.
Overview of Argon's Electronic Configuration

Argon's electronic configuration is [Ne] 3s² 3p⁶. This configuration indicates that the outermost energy level of argon is fully occupied, which makes it stable and unreactive. The full outer energy level also means that argon does not readily form ions or participate in chemical bonding.
Factors Influencing Argon's Reactivity

Several factors influence argon's reactivity, including:
- Electronegativity: Argon has a low electronegativity value of 0.0, which indicates that it does not readily attract electrons. This low electronegativity value makes it difficult for argon to form covalent bonds with other elements.
- Ionization energy: Argon has a high ionization energy of 1520.5 kJ/mol, which means that it requires a significant amount of energy to remove an electron from an argon atom. This high ionization energy makes it difficult for argon to form ions or participate in chemical reactions.
- Electron affinity: Argon has a negative electron affinity of -1.77 eV, which indicates that it does not readily accept electrons. This negative electron affinity value makes it difficult for argon to form anions or participate in chemical reactions.
Argon's Bonding Tendency with Other Elements

Despite its low reactivity, argon can exhibit some degree of bonding tendency with other elements under certain conditions. Here are some examples:
- Hydrogen: Argon can form a compound with hydrogen, known as argon hydride (ArH⁺). This compound is formed when argon is reacted with hydrogen in the presence of an electric discharge.
- Oxygen: Argon can form a compound with oxygen, known as argon oxide (ArO⁺). This compound is formed when argon is reacted with oxygen in the presence of an electric discharge.
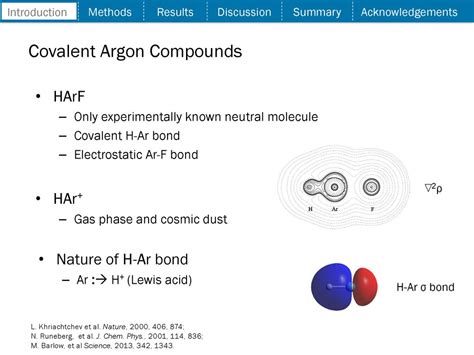
- Fluorine: Argon can form a compound with fluorine, known as argon fluoride (ArF). This compound is formed when argon is reacted with fluorine in the presence of an electric discharge.
- Carbon: Argon can form a compound with carbon, known as argon carbide (ArC⁺). This compound is formed when argon is reacted with carbon in the presence of an electric discharge.
Applications of Argon's Bonding Tendency

Argon's bonding tendency has several applications in various fields, including:
- Lighting: Argon is used in lighting fixtures, such as fluorescent lamps and plasma TVs, due to its ability to form excited states that emit light.
- Welding: Argon is used as a shielding gas in welding processes, such as TIG and MIG welding, due to its ability to protect the weld area from atmospheric gases.
- Insulation: Argon is used as a filler gas in insulation materials, such as fiberglass and rock wool, due to its low thermal conductivity and ability to reduce heat transfer.
- Semiconductors: Argon is used in the production of semiconductors, such as silicon and germanium, due to its ability to form compounds with these elements.
Conclusion
In conclusion, argon's bonding tendency with other elements is influenced by several factors, including electronegativity, ionization energy, and electron affinity. Despite its low reactivity, argon can exhibit some degree of bonding tendency with other elements under certain conditions. The applications of argon's bonding tendency are diverse and include lighting, welding, insulation, and semiconductors.
We hope this article has provided you with a comprehensive understanding of argon's bonding tendency with other elements. If you have any questions or comments, please feel free to share them with us.
What is the electronic configuration of argon?
+The electronic configuration of argon is [Ne] 3s² 3p⁶.
What are the factors that influence argon's reactivity?
+The factors that influence argon's reactivity include electronegativity, ionization energy, and electron affinity.
What are some examples of argon's bonding tendency with other elements?
+Some examples of argon's bonding tendency with other elements include argon hydride (ArH⁺), argon oxide (ArO⁺), argon fluoride (ArF), and argon carbide (ArC⁺).