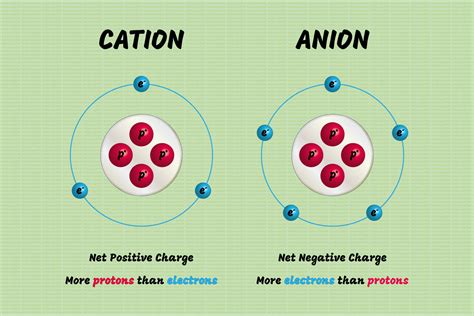
Metals are a class of elements that are known for their ability to form cations, which are positively charged ions. This phenomenon is a fundamental aspect of chemistry, and it plays a crucial role in many chemical reactions and processes. But why do metals form cations in the first place? To understand this, we need to delve into the world of atomic structure and chemical bonding.

Atomic Structure and Electrons
To understand why metals form cations, we need to start with the atomic structure of metals. Metals are a class of elements that are characterized by their ability to lose electrons easily. This is because metal atoms have a relatively small number of electrons in their outermost energy level, which is also known as the valence shell.
The valence shell is the outermost energy level of an atom, and it is where the electrons that participate in chemical bonding are located. In metal atoms, the valence shell is not fully occupied, which means that there are not enough electrons to fill the available energy levels. This makes it easy for metal atoms to lose electrons, which is a key factor in the formation of cations.
Electron Configuration and Ionization Energy
The electron configuration of a metal atom is also an important factor in the formation of cations. Metal atoms tend to have a low ionization energy, which is the energy required to remove an electron from an atom. This is because the electrons in the valence shell of a metal atom are not strongly attracted to the nucleus, which makes it easy to remove them.
When a metal atom loses an electron, it forms a cation, which is a positively charged ion. The cation has a positive charge because it has lost an electron, which has a negative charge. The positive charge on the cation is balanced by the negative charge on the electron that was lost.
Chemical Bonding and Cation Formation
Chemical bonding is another important factor in the formation of cations. When a metal atom bonds with a nonmetal atom, it forms an ionic bond, which is a type of chemical bond that involves the transfer of electrons.
In an ionic bond, the metal atom loses one or more electrons to the nonmetal atom, which forms a cation. The nonmetal atom gains one or more electrons, which forms an anion, which is a negatively charged ion. The cation and anion are attracted to each other, which forms the ionic bond.

Examples of Cation Formation
There are many examples of cation formation in chemistry. One common example is the reaction between sodium metal and chlorine gas. When sodium metal is reacted with chlorine gas, it forms sodium chloride, which is also known as table salt.
In this reaction, the sodium metal loses an electron to the chlorine gas, which forms a cation. The chlorine gas gains an electron, which forms an anion. The cation and anion are attracted to each other, which forms the ionic bond.
Sodium (Na) → Na+ (cation) + e- (electron) Chlorine (Cl2) + e- (electron) → Cl- (anion)
Conclusion
In conclusion, metals form cations because of their atomic structure and chemical bonding properties. Metal atoms have a relatively small number of electrons in their outermost energy level, which makes it easy for them to lose electrons. When a metal atom loses an electron, it forms a cation, which is a positively charged ion.
Chemical bonding is also an important factor in the formation of cations. When a metal atom bonds with a nonmetal atom, it forms an ionic bond, which involves the transfer of electrons. The metal atom loses one or more electrons to the nonmetal atom, which forms a cation.
We hope this article has helped you understand why metals form cations. If you have any questions or comments, please leave them in the section below.

What is Ionization Energy?
Ionization energy is the energy required to remove an electron from an atom. It is an important factor in the formation of cations, as it determines how easily an electron can be removed from an atom.

How is Ionization Energy Measured?
Ionization energy is typically measured in units of electronvolts (eV) or joules (J). It can be measured using a variety of techniques, including spectroscopy and mass spectrometry.
What is Electronegativity?
Electronegativity is a measure of an atom's ability to attract electrons. It is an important factor in the formation of cations, as it determines how easily an atom can lose electrons.

How is Electronegativity Measured?
Electronegativity is typically measured using a variety of scales, including the Pauling scale and the Mulliken scale. It can be measured using a variety of techniques, including spectroscopy and X-ray photoelectron spectroscopy.
Why do metals form cations?
+Metals form cations because of their atomic structure and chemical bonding properties. Metal atoms have a relatively small number of electrons in their outermost energy level, which makes it easy for them to lose electrons.
What is ionization energy?
+Ionic energy is the energy required to remove an electron from an atom. It is an important factor in the formation of cations, as it determines how easily an electron can be removed from an atom.
What is electronegativity?
+Electronegativity is a measure of an atom's ability to attract electrons. It is an important factor in the formation of cations, as it determines how easily an atom can lose electrons.