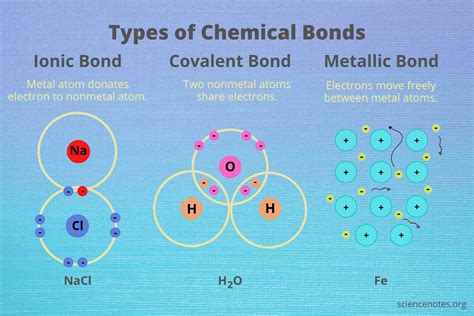
Ionic bonds are a crucial concept in chemistry, forming the foundation of many chemical compounds. In this article, we will delve into the world of ionic bonds, exploring the elements that form them, their characteristics, and the ways in which they are created.
At the heart of ionic bonding lies the transfer of electrons between atoms. This transfer of electrons results in the formation of ions with opposite charges, which are then attracted to each other, creating a strong chemical bond. But which elements are capable of forming these ionic bonds? To answer this question, we need to look at the periodic table and the properties of the elements.

Elements That Form Ionic Bonds
Ionic bonds typically form between two types of elements: metals and nonmetals. Metals, found on the left side of the periodic table, tend to lose electrons easily, resulting in the formation of positively charged ions (cations). Nonmetals, found on the right side of the periodic table, tend to gain electrons easily, resulting in the formation of negatively charged ions (anions).
Metals
Metals are a group of elements that are known for their ability to conduct electricity and heat. They are typically shiny, malleable, and ductile. Some examples of metals that commonly form ionic bonds include:
- Alkali metals (Group 1): Lithium (Li), Sodium (Na), Potassium (K), Rubidium (Rb), Caesium (Cs), and Francium (Fr)
- Alkaline earth metals (Group 2): Magnesium (Mg), Calcium (Ca), Strontium (Sr), Barium (Ba), Radium (Ra), and Beryllium (Be)
- Transition metals: Iron (Fe), Copper (Cu), Silver (Ag), and Gold (Au)
These metals tend to lose one or more electrons to form a positive ion. For example, when sodium (Na) loses an electron, it forms a sodium ion (Na+) with a +1 charge.

Nonmetals
Nonmetals are a group of elements that are typically dull, brittle, and poor conductors of electricity. They are found on the right side of the periodic table and tend to gain electrons to form a negative ion. Some examples of nonmetals that commonly form ionic bonds include:
- Halogens (Group 17): Fluorine (F), Chlorine (Cl), Bromine (Br), Iodine (I), and Astatine (At)
- Noble gases (Group 18): Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe), and Radon (Rn)
- Oxygen (O), Nitrogen (N), and Carbon (C)
These nonmetals tend to gain one or more electrons to form a negative ion. For example, when chlorine (Cl) gains an electron, it forms a chloride ion (Cl-) with a -1 charge.

Characteristics of Ionic Bonds
Ionic bonds have several characteristics that distinguish them from other types of chemical bonds. Some of the key characteristics of ionic bonds include:
- Electrostatic attraction: Ionic bonds are formed through the electrostatic attraction between positively charged cations and negatively charged anions.
- Strong and rigid: Ionic bonds are typically strong and rigid, resulting in high melting and boiling points.
- Brittle: Ionic compounds tend to be brittle and prone to cracking or shattering.
- Conductors: Ionic compounds are typically poor conductors of electricity, but they can conduct electricity when molten or dissolved in water.

Formation of Ionic Bonds
Ionic bonds are formed through the transfer of electrons between atoms. This transfer of electrons results in the formation of ions with opposite charges, which are then attracted to each other, creating a strong chemical bond.
The formation of ionic bonds can be represented by the following equation:
A + B → AB
Where A is the metal atom, B is the nonmetal atom, and AB is the resulting ionic compound.
For example, when sodium (Na) reacts with chlorine (Cl), the following reaction occurs:
2Na (s) + Cl2 (g) → 2NaCl (s)
In this reaction, sodium loses an electron to form a sodium ion (Na+), while chlorine gains an electron to form a chloride ion (Cl-). The resulting sodium chloride (NaCl) compound is an ionic compound with a strong electrostatic attraction between the positively charged sodium ions and the negatively charged chloride ions.

Importance of Ionic Bonds
Ionic bonds play a crucial role in the formation of many chemical compounds. They are found in a wide range of substances, from table salt (sodium chloride) to the minerals that make up the Earth's crust.
Ionic bonds are also important in biology, where they play a key role in the formation of biomolecules such as proteins, carbohydrates, and nucleic acids.
In addition, ionic bonds are used in a variety of industrial applications, including the production of ceramics, glass, and concrete.

Conclusion
In conclusion, ionic bonds are a fundamental concept in chemistry, forming the basis of many chemical compounds. By understanding the elements that form ionic bonds, their characteristics, and the ways in which they are created, we can gain a deeper appreciation for the complex and fascinating world of chemistry.
If you have any questions or comments about ionic bonds, please leave them in the section below. We would love to hear from you!
What is an ionic bond?
+An ionic bond is a type of chemical bond that forms between two atoms through the transfer of electrons, resulting in the formation of ions with opposite charges.
Which elements form ionic bonds?
+Metal and nonmetal elements typically form ionic bonds. Metals tend to lose electrons to form positively charged ions, while nonmetals tend to gain electrons to form negatively charged ions.
What are the characteristics of ionic bonds?
+Ionic bonds are strong and rigid, resulting in high melting and boiling points. They are also brittle and prone to cracking or shattering.