The concept of chemical reactions is fundamental to various fields, including chemistry, biology, and physics. One of the most intriguing aspects of chemical reactions is the formation of products. In this article, we will explore three ways to form one product in a reaction, delving into the underlying principles and mechanisms.
Understanding Chemical Reactions

Chemical reactions involve the transformation of one or more substances into new substances. This process is often represented by a chemical equation, which uses symbols and formulas to describe the reactants, products, and reaction conditions. The product of a reaction is the substance formed as a result of the reaction.
The Importance of Product Formation
Product formation is a crucial aspect of chemical reactions, as it allows us to understand the outcome of a reaction and predict the properties of the resulting substance. In many cases, the product of a reaction is the desired outcome, such as in the production of pharmaceuticals, fuels, or other chemicals.
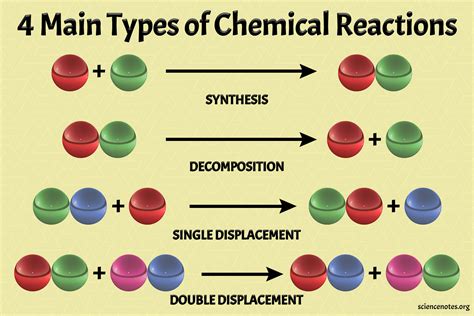
Method 1: Synthesis Reactions

Synthesis reactions involve the combination of two or more reactants to form a single product. This type of reaction is often represented by the general equation:
A + B → C
In this equation, A and B are the reactants, and C is the product.
Example: Formation of Water
A classic example of a synthesis reaction is the formation of water from hydrogen and oxygen gases:
2H2 + O2 → 2H2O
In this reaction, hydrogen gas (H2) and oxygen gas (O2) combine to form water (H2O).
Method 2: Decomposition Reactions

Decomposition reactions involve the breakdown of a single reactant into two or more products. This type of reaction is often represented by the general equation:
A → B + C
In this equation, A is the reactant, and B and C are the products.
Example: Decomposition of Hydrogen Peroxide
A common example of a decomposition reaction is the breakdown of hydrogen peroxide (H2O2) into water and oxygen:
2H2O2 → 2H2O + O2
In this reaction, hydrogen peroxide decomposes into water and oxygen gas.
Method 3: Replacement Reactions

Replacement reactions involve the replacement of one reactant with another reactant to form a new product. This type of reaction is often represented by the general equation:
A + BC → AC + B
In this equation, A and BC are the reactants, and AC and B are the products.
Example: Replacement of Zinc with Copper
A classic example of a replacement reaction is the replacement of zinc with copper in a solution of copper sulfate:
Zn + CuSO4 → ZnSO4 + Cu
In this reaction, zinc metal replaces copper ions in the copper sulfate solution to form zinc sulfate and copper metal.
Conclusion: Forming One Product in a Reaction
In conclusion, there are three ways to form one product in a reaction: synthesis reactions, decomposition reactions, and replacement reactions. Each of these methods involves a unique set of reactants and reaction conditions that ultimately lead to the formation of a single product.
We hope this article has provided you with a deeper understanding of the principles and mechanisms underlying product formation in chemical reactions. Whether you're a student, researcher, or simply curious about chemistry, we encourage you to explore further and discover the many wonders of chemical reactions.
If you have any questions or comments, please don't hesitate to share them below. Your feedback is valuable to us, and we look forward to hearing from you.
What is a synthesis reaction?
+A synthesis reaction is a type of chemical reaction in which two or more reactants combine to form a single product.
What is a decomposition reaction?
+A decomposition reaction is a type of chemical reaction in which a single reactant breaks down into two or more products.
What is a replacement reaction?
+A replacement reaction is a type of chemical reaction in which one reactant replaces another reactant to form a new product.